Widespread Colonisation of Tanzanian Catchments by Introduced Oreochromis Tilapia Fishes: the Legacy from Decades of Deliberate Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oreochromis Rukwaensis) Ecological Risk Screening Summary
Lake Rukwa Tilapia (Oreochromis rukwaensis) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2012 Revised, July 2018 Web Version, 6/4/2020 Organism Type: Fish Overall Risk Assessment Category: Uncertain 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Africa: Lake Rukwa in Tanzania.” From Shechonge et al. (2019): “Oreochromis rukwaensis (Hilgendorf & Pappenheim 1903) previously known only from Lake Rukwa was present in an upstream section of the Ruaha river system, where a major exploited population was recorded at the Mtera Dam Lake [Tanzania].” Status in the United States No records of Oreochromis rukwaensis occurrences in the United States were found. No information on trade of O. rukwaensis in the United States was found. 1 The Florida Fish and Wildlife Conservation Commission has listed the tilapia, Oreochromis rukwaensis as a prohibited species. Prohibited nonnative species (FFWCC 2020), "are considered to be dangerous to the ecology and/or the health and welfare of the people of Florida. These species are not allowed to be personally possessed or used for commercial activities." Means of Introductions in the United States No records of Oreochromis rukwaensis occurrences in the United States were found. Remarks No additional remarks. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Eschmeyer et al. (2018), Oreochromis rukwaensis (Hilgendorf and Pappenheim 1903) is the current valid name of this species. From ITIS (2018): Kingdom Animalia -

Lake Chala Tilapia (Oreochromis Hunteri) Ecological Risk Screening Summary
Lake Chala Tilapia (Oreochromis hunteri) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2012 Revised, June 2018 Web Version, 12/15/2020 Organism Type: Fish Overall Risk Assessment Category: Uncertain Photo: D. H. Eccles. Licensed under Creative Commons BY-NC 3.0. Available: http://www.fishbase.org/photos/PicturesSummary.php?StartRow=0&ID=2032&what=species&T otRec=2. (June 18, 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018a): “Africa: endemic to Lake Chala [Seegers et al. 2003].” 1 Status in the United States No records of Oreochromis hunteri in trade or in the wild in the United States were found. The Florida Fish and Wildlife Conservation Commission has listed the tilapia Oreochromis hunteri as a prohibited species. Prohibited nonnative species (FFWCC 2018), "are considered to be dangerous to the ecology and/or the health and welfare of the people of Florida. These species are not allowed to be personally possessed or used for commercial activities. All species in the genus Oreochromis are considered regulated Type A species in Washington. Regulated Type A species (Washington State Senate 2019) are “nonnative aquatic animal species that pose a low to moderate invasive risk that can be managed based on intended use or geographic scope of introduction, have a beneficial use, and are a priority for department-led or department-approved management of the species' beneficial use and invasive risks.” Possession of any species of tilapia is prohibited without permit in the State of Louisiana (Louisiana State Legislature 2019). O. amphimelas falls within Group I of New Mexico’s Department of Game and Fish Director’s Species Importation List (New Mexico Department of Game and Fish 2010). -

A BIBLIOGRAPHY of IMPORTANT TILAPIAS (PISCES: CICHLIDAE) for AQUACULTURE Oreochromisvariabilis, 0 Andersoni, 0
AMV'__ BIBLIOGRAPHIES 6 A BIBLIOGRAPHY OF IMPORTANT TILAPIAS (PISCES: CICHLIDAE) FOR AQUACULTURE Oreochromisvariabilis, 0 andersoni, 0. esculentus, 0. leucostictus, 0. rortimer, 0. spilurus niger,Sarotherodon melanotheron and Tilapia sparnmani PETER SCHOENEN INTERNATIONAL CENTER FOR LIVING AQUATIC RESOURCES MANAGEMENT A BIBLIOGRAPHY OF IMPORTANT TILAPIAS (PISCES: CICHLIDAE) FOR AQUACULTURE Oreochromls variabilis, 0. andersoni, 0. esculentus, 0. leucostictus, 0. mortimeri, 0. spilurus niger, Saro therodon melano theron and Tilapia sparrmanii Peter Schoenen International Collection "Cichlid Papers" The Referencc Service Parkstr. 15 D-5176 Inden 4 Federal Republic of Germany 1985 INTERNATIONAL CENTER FOR LIVING AQUATIC RESOURCES MANAGEMENT MANILA, PHILIPPINES A bibliography of important tilapias (Pisces: Cichlidae) for aquaculture Oreochromis variabilis, 0. andersonii, 0. esculentus, 0. leucostictus, 0. mort/tmer, 0. spilunis niger, Sarotherodon melanothero,, ard -/ilapiasparrmanii PETER SCHOENEN Published by the International Center for Living Aquatic Resources Management, MCC P.O. Box 1501, Makati, Metro Manila, Philippines with financial assistance from the International Development Research Centre of Canada through ICLARM's Selective Information Service project. 1985 Printed in Manila, Philippins This bibliography is produced directly from the author's manuscript in oider to provide tilapia workers with a useful document in the shortest time. The author should be consulted in the event of difficulty ir verifying details of particular references or in locating sources. ISSN 0115-5997 ISBN 971-1022-19-2 Schoenen, P. 1985, A bibliography of important tilapias (Pisces: Cichlidae) for aquaculture Oreochromis variabilis, 0. andersonii, 0. esculentus, 0. leucostictus, 0. mortimeri, 0. spilurut niger, Sarotherodon mela. notheron and Tilapia sparrrnanii. ICLAHM Biblio graphies 6,99 p. International Center for Living Aquatic Resources Management, Manila, Philippines. -

Implications for Management AFRICAN GREAT LAKES
AFRICAN GREAT LAKES CONFERENCE 2nd – 5th MAY 2017, ENTEBBE, UGANDA Dynamics of Fish Stocks of Commercial Importance in Lake Victoria, East Africa: Implications for Management Robert Kayanda, Anton Taabu-Munyaho, Dismas Mbabazi, Hillary Mrosso, and Chrisphine Nyamweya INTRODUCTION • Lake Victoria with a surface area of 68,800 sqkm is the world’s second largest freshwater body • It supports one of the world’s most productive inland fisheries with the estimated total fish landings from the lake for the period of 2011 to 2014 have been about 1 million tons with a beach value increasing from about US$ 550 Million in 2011 to about US$ 840 million in 2014. • It supports about 220,000 fishers (Frame Survey 2016) • The fish stocks of Lake Victoria have changed dramatically since the introduction of Nile perch Lates niloticus during the late 1950s and early 1960s Fishery Haplochromines The Original Fish Fauna Brycinus sp Protopterus Rastrineobola Mormyrus spp Barbus spp Bagrus docmac Labeo Schilbe intermedius Oreochromis variabilis Clarias gariepinus Mormyrus spp Synodontis victoriae Oreochromis leucostictus INTRODUCTION Currently, the fisheries is dominated by four major commercial important species, these are; •Nile perch •Dagaa •Nile tilapia •Haplochromis Apart from Nile tilapia only estimated through trawl and catch surveys, the other 3 are estimated through trawl, acoustics, and catch INTRODUCTION This paper summarizes current knowledge of the status of the fish stocks and reviews the need for species specific management plans for the major commercial important fish species of Lake Victoria (Nile perch, Nile tilapia, dagaa and haplochromines). Methods • Fisheries dependent – Frame surveys – Catch assessment surveys • Fisheries independent – Acoustic – Bottom trawl Biomass and relative abundance • Total biomass from the surveys 3500 remained fairly stable over time. -

Pangani Basin: a Situation Analysis
Pangani Basin: A Situation Analysis IUCN Eastern Africa Programme 2003 i Published by: Copyright: © 2003 International Union for Conservation of Nature and Natural Resources This publication may be produced in whole or part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgement of the source is made. IUCN would appreciate receiving a copy of any publication which uses this publication as a source. No use of this publication may be made for resale or other commercial purpose without the prior written permission of IUCN. Citation: IUCN Eastern Africa Programme, 2003. The Pangani River Basin: A Situation Analysis, xvi + 104pp. ISBN: 2-8317-0760-9 Design and layout: Gordon O. Arara Printed by: ScanHouse Press Ltd. Photo 1: The summit of Mount Kilimanjaro; Photo 2: Forest stand at 1 Shire Njoro; Photo 3: Gate controlling the release of water into irrigation furrows; Photo 4: Children swimming in an irrigation 3 4 reservoir; Photo 5: Sisal plantations; Photo 6: Irrigated rice scheme; 2 Photo 7: Water gauging station at Chemka Spring; Photo 8: Vandalized gate controlling the release of water into irrigation furrows; Photo 9: 5 Dam wall at Nyumba ya Mungu Reservoir (color changes mark the declining water levels); Photo 10: A vendor sells water from a borehole 6 9 10 Photos 1, 3, 5, 6, 8, 9 copyright 2003 Kelly West; Photos 2, 7 7 8 copyright 2002 Kim Geheb; Photos 4, 10 copyright 2003 Ger Bergkamp. Available from: IUCN- EARO Publications Service Unit, P. O. Box 68200 - 00200, Nairobi, Kenya; Telephone ++ 254 20 890605-12; Fax ++ 254 20 890615; E-mail: [email protected] The designations of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of the participating organiza- tions concerning the legal status of any country, territory, or area, or of its authorities, or con- cerning the delimitation of its frontiers or boundaries. -

Northern Tanzania Embodies What Is for Many Mt Kilimanjaro the Quintessential Africa
©Lonely Planet Publications Pty Ltd N o r t h e r n T a n z a n i a Why Go? For many visitors to Tanzania, it’s all about the north. With Moshi..............................148 snow-capped Mt Kilimanjaro, wildlife-packed Ngorongoro Machame .......................153 Crater, red-cloaked Maasai warriors and the vast plains of Marangu ........................ 154 the Serengeti, northern Tanzania embodies what is for many Mt Kilimanjaro the quintessential Africa. But there’s much more to this ma- National Park ................ 156 jestic and mythical place and it would draw scores of visitors Arusha ............................161 even if it didn’t host these African icons. Arusha National Park ....176 Crater-capped Mt Meru is a climb that rivals its taller Tarangire neighbour, dry-season wildlife watching in Tarangire Na- National Park .................181 tional Park is as good as any other park in Africa, and the Lake Manyara desolate Rift Valley landscape between Lakes Manyara and National Park ................ 183 Natron will mesmerise you. Sleep in a coff ee plantation, Lake Natron .................. 186 hunt with modern-day nomads, ride camels, canoe with hip- Ngorongoro pos…well, you get the point. Conservation Area ........ 189 You couldn’t possibly do it all in one trip, but you’ll make a lifetime of memories no matter how much time you have. Lake Eyasi ..................... 194 Serengeti National Park ................ 195 When to Go Best of Culture Arusha » Cultural Tourism Programs °C/°F Temp Rainfall inches/mm (p 168 ) 40/104 16/400 » Lake Eyasi (p 194 ) 30/86 12/300 » Coffee Tours (p 149 ) 20/68 8/200 » The Maasai (p 178 ) 10/50 4/100 Best of Nature 0/32 0 J FDNOSAJJMAM » Serengeti National Park (p 195 ) Jan-Mar The Apr-May Rain Sep-Oct The best » The Crater Highlands (p 191 ) wildebeest turns roads time to travel. -

Ngorongoro Conservation Area, Wildlife Reserve Within World's
Sentinel Vision EVT-439 Ngorongoro Conservation Area, wildlife reserve within 29 April 2019 World's largest crater Sentinel-2 MSI acquired on 16 September 2018 at 07:36:09 UTC Sentinel-2 MSI acquired on 24 September 2018 at 07:46:31 UTC Sentinel-1 CSAR IW acquired on 28 December 2018 at 15:55:36 UTC Author(s): Sentinel Vision team, VisioTerra, France - [email protected] 2D Layerstack Keyword(s): volcano caldera, canyon, UNESCO World heritage, biodiversity, Great African rift, Gregory rift, Tanzania Fig. 1 - S2 (16 & 24.09.2018) - 4,3,2 natural colour - Ngorongoro Conservation Area is a green island within a drier landscape. 2D view Ngorongoro Conservation Area is not only the spectacular landscape of several extensive volcano calderas nested along the Great African rift; it is also an oasis of life encompassing rare large mammals that sustains even during the dry season. UNESCO listed this spectacular location as a World Heritage site, its sheet notes: "The Ngorongoro Conservation Area (809,440 ha) spans vast expanses of highland plains, savanna, savanna woodlands and forests, from the plains of the Serengeti National Park in the north-west, to the eastern arm of the Great Rift Valley. The area was established in 1959 as a multiple land use area, with wildlife coexisting with semi-nomadic Maasai pastoralists practising traditional livestock grazing. It includes the spectacular Ngorongoro Crater, the world's largest caldera, and Olduvai Gorge, a 14km long deep ravine. The property has global importance for biodiversity conservation in view of the presence of globally threatened species such as the black Rhino, the density of wildlife inhabiting the Ngorongoro Crater and surrounding areas throughout the year, and the annual migration of wildebeest, zebra, Thompson's and Grant's gazelles and other ungulates into the northern plains." Overlook on the rim of Ngorongoro Crater, Tanzania - Source: Thomas Huston. -

List of Rivers of Tanzania
Sl.No Name Draining Into 1 Bubu River Endorheic basins 2 Deho River East Coast 3 Great Ruaha River East Coast 4 Ifume River Congo Basin 5 Ipera River East Coast 6 Isanga River Nile basin 7 Jipe Ruvu River East Coast 8 Kagera River Nile basin 9 Kalambo River Congo Basin 10 Kavuu River Endorheic basins 11 Kihansi East Coast 12 Kikafu River East Coast 13 Kikuletwa River East Coast 14 Kimani River East Coast 15 Kimbi River East Coast 16 Kiseru River East Coast 17 Kizigo River East Coast 18 Kolungazao River East Coast 19 Lake Burunge Endorheic basins 20 Lake Eyasi Endorheic basins 21 Lake Jipe East Coast 22 Lake Malawi Zambezi basin 23 Lake Manyara Endorheic basins 24 Lake Natron Endorheic basins 25 Lake Rukwa Endorheic basins 26 Lake Tanganyika Congo Basin 27 Lake Victoria Nile basin 28 Little Ruaha River East Coast 29 Loasi River Congo Basin 30 Luamfi River Congo Basin 31 Luega River Congo Basin 32 Luegele River Congo Basin 33 Luengera River East Coast 34 Luhombero River East Coast 35 Lukigura River East Coast 36 Lukosi River East Coast 37 Lukuledi River East Coast 38 Lukumbule River East Coast 39 Lukwika River East Coast 40 Lungonya River East Coast 41 Luwegu River East Coast 42 Malagarasi River Congo Basin 43 Mara River Nile basin 44 Matandu River East Coast 45 Mavuji River East Coast 46 Mbarali River East Coast 47 Mbiki River East Coast 48 Mbungu River East Coast 49 Mbwemkuru River East Coast www.downloadexcelfiles.com 50 Mgeta River East Coast 51 Migasi River East Coast 52 Miyombo River East Coast 53 Mkata River East Coast 54 Mkomazi -

Trophic Niche Segregation in the Nilotic Ichthyofauna of Lake Albert (Uganda, Africa)
Environmental Biology of Fishes (2005) 74:247–260 Ó Springer 2005 DOI 10.1007/s10641-005-3190-8 Trophic niche segregation in the Nilotic ichthyofauna of Lake Albert (Uganda, Africa) Linda M. Campbella,d, Sylvester B. Wanderab, Robert J. Thackerc,e, D. George Dixona & Robert E. Heckya aDepartment of Biology, University of Waterloo, 200 University Avenue. Waterloo, Ontario, Canada N2L 3G1 bFisheries Resources Research Institute, P.O. Box 343, Jinja, Uganda cDepartment of Physics and Astronomy, McMaster University, 1280 Main St. W, Hamilton, Ontario, Canada dCurrent address: School of Environmental Studies and Department of Biology, Queen’s University, Kingston, ON, Canada K7L 3N6 (e-mail: [email protected]) eCurrent address: Department of Physics and Astronomy, Queen’s University, Kingston, ON, Canada K7L 3N6 Received 29 April 2004 Accepted 13 February 2005 Key words: d13C, d15N, food webs, Nile perch, stable isotopes Synopsis Nile perch, Lates niloticus, and Nile tilapia, Oreochromis niloticus, were originally transplanted from Lake Albert in western Uganda to the African Great Lakes, Lake Victoria and Lake Kyoga, where they are partially implicated in reduction of the fish species diversity. Lake Albert is facing multiple environmental changes, including declining fish species diversity, hyper-eutrophication, hypoxia, and reduced fish catches. To examine the role of Nile perch and Nile tilapia in the food web in their native Lake Albert, we estimated their diets using stable nitrogen and carbon isotopes. In Lake Albert, the tilapiine congeners (closely related species), Tilapia zillii, Oreochromis leucostictus, and Sarethorodon galilaeus, and the centropomid Nile perch congener, Lates macrophthalmus, have narrower diet breath in the presence of the native O. -
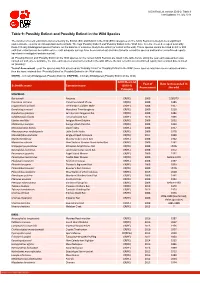
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2019-2: Table 9 Last Updated: 18 July 2019 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded in -

The Status and Future Prospects of Hydropower for Sustainable Water and Energy Development in Tanzania
Hindawi Journal of Renewable Energy Volume 2018, Article ID 6570358, 12 pages https://doi.org/10.1155/2018/6570358 Review Article The Status and Future Prospects of Hydropower for Sustainable Water and Energy Development in Tanzania Baraka Kichonge Mechanical Engineering Department, Arusha Technical College (ATC), P.O. Box 296, Arusha, Tanzania Correspondence should be addressed to Baraka Kichonge; [email protected] Received 7 January 2018; Accepted 8 March 2018; Published 6 May 2018 Academic Editor: Wei-Hsin Chen Copyright © 2018 Baraka Kichonge. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Tanzania is among the countries with the fastest growing economy in Africa and therefore the need for afordable, clean, and most importantly sustainable electrical energy to meet her ever growing demands is pressing. In recent years, the country’s electricity needs have been largely dominated by thermal generations despite the fact that Tanzania is gifed with huge hydropower resource potential approximated at 38,000 MW with only a very small portion exploited to date. However, the exploited potential is expected to grow by commissioning of identifed large and medium-scale hydropower projects with a total installed capacity of 4,765 MW currently under various stages of implementation. Moreover, the geographical location of Tanzania has several benefts to support development of small hydropower projects essential for appropriate utilization of available water resources as a way of mitigating climate challenges efects. Over the last decade, the country electricity demand along with end-use of energy has witnessed signifcant increases as economic development spreads towards achieving Vision 2025 goals. -

Diversity of Plant Niches Available for Hominin Settlement During Upper Bed I- Lower Bed II: a Phytolith Perspective, Oldupai Gorge (Tanzania)
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2020-01-09 Diversity of plant niches available for Hominin settlement during Upper Bed I- Lower Bed II: A phytolith perspective, Oldupai Gorge (Tanzania) Itambu, Makarius Peter Itambu, M. P. (2020). Diversity of plant niches available for Hominin settlement during Upper Bed I- Lower Bed II: A phytolith perspective, Oldupai Gorge (Tanzania) (Unpublished doctoral thesis). University of Calgary, Calgary, AB. http://hdl.handle.net/1880/111642 doctoral thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Diversity of plant niches available for Hominin settlement during Upper Bed I- Lower Bed II: A phytolith perspective, Oldupai Gorge (Tanzania) by Makarius Peter Itambu A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN ARCHAEOLOGY CALGARY, ALBERTA JANUARY, 2020 © Makarius Peter Itambu 2020 ABSTRACT This research focused on reconstructing the diversity of plant landscapes that framed hominin evolution in Oldupai Gorge. The study had two main overarching goals with interrelated objectives. The first goal was to assess the synergetic links between hominin habitats and ecological preferences during the Pleistocene at Oldupai Gorge, and the second was the reconstruction of vegetation patterns characteristic during Upper Most Bed I to Lower Most Bed II.