Declaration of Blake Follis 1 2 3 4 5 6 7 8 9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Modoc Nation (Formerly Known As the “Modoc Tribe”), A
The Modoc Nation (formerly known as the “Modoc Tribe”), a federally recognized native nation by virtue of the Lakes Treaty of 1864 and the Klamath Tribe Restoration Act of 1986 We can no longer tolerate outright refusal of protecting our best interest. If the current conditions were to continue the outright extermination of our people would be complete. Our Ancestral land and water rights will be non- existent and we will be unable to hunt fish and gather as we have always done since time immemorial. We have always been the Modoc Tribe of Northern California and Southern Oregon. We have always been a separate tribe, we were never a band of the Klamath’s and we have never relinquished our Federal Recognition. Our Tribe existed long before there was a California or Oregon. Our tribe is a Federally Recognized Tribe, instead of taking things out of context and stating we are not separate from the Klamath Tribe. (Although the short terminology refers to the Klamath Tribe or Tribes, the long definition has always stated 3 separate tribes in all treaties). There is the Modoc Tribe of Oklahoma, who was forced there not by choice and chose to stay rather than be forced to live with the Klamath tribe. There are Modocs enrolled in the Klamath Tribe(s) as “Klamath”, since the Klamath tribe refuses to acknowledge the Modoc tribe (except when there is financial gain by using our name). We represent all Modoc people of Northern California and Southern Oregon who are enrolled in The Modoc Nation as “Modoc” as we are the Modoc Tribe who have existed in our Ancestral Homelands for over fourteen thousand years or more. -

In the Recent Dear Colleague Letter 99-30, OCSE Notified You of A
Location Codes Workgroup FIPS Coding Scheme Recommendation Summary Position 1 Position 2 Positions 3-5 Interstate Case FIPS State Identifier County/Functional Entity 9 0 BIA Tribe Identifier Tribal Case (Federally recognized) 8 0 ISO Country Identifier International Case Exception 0-9, A-Z (Canada – sub- jurisdiction) Tribal and International Case Location Codes 1 OCSE Case Locator Code Data Standards Tribal locator codes coding scheme Tribal Case Locator Codes • Classification code - 9 in position 1 • “0”(zero) in position 2 • Tribe Identification - BIA code in positions 3-5 Example: Chickasaw Nation 90906 • Addresses for tribal grantees– provided by tribes to IRG staff List of current tribal grantees: http://ocse.acf.hhs.gov/int/directories/index.cfm?fuseaction=main.tribalivd • Link to tribal government addresses web site: http://www.doi.gov/leaders.pdf 11/15/2006 2 OCSE Case Locator Code Data Standards Tribal Identification Codes Code Name 001 Eastern Band of Cherokee Indians of North Carolina 006 Onondaga Nation of New York 007 St. Regis Band of Mohawk Indians of New York 008 Tonawanda Band of Seneca Indians of New York 009 Tuscarora Nation of New York 011 Oneida Nation of New York 012 Seneca Nation of New York 013 Cayuga Nation of New York 014 Passamaquoddy Tribe of Maine 018 Penobscot Tribe of Maine 019 Houlton Band of Maliseet Indians of Maine 020 Mashantucket Pequot Tribe of Connecticut 021 Seminole Tribe of Florida, Dania, Big Cypress, Brighton, Hollywood & Tampa Reservations 026 Miccosukee Tribe of Indians of Florida 027 Narragansett -

Indian Lands of Federally Recognized Tribes of the United States
132°W 131°W 130°W 129°W 128°W 127°W 126°W 125°W 124°W 123°W 122°W 121°W 120°W 119°W 118°W 117°W 116°W 115°W 114°W 113°W 112°W 111°W 110°W 109°W 108°W 107°W 106°W 105°W 104°W 103°W 102°W 101°W 100°W 99°W 98°W 97°W 96°W 95°W 94°W 93°W 92°W 91°W 90°W 89°W 88°W 87°W 86°W 85°W 84°W 83°W 82°W 81°W 80°W 79°W 78°W 77°W 76°W 75°W 74°W 73°W 72°W 71°W 70°W 69°W 68°W 67°W 66°W 65°W 64°W 63°W 48°N 46°N 47°N Neah Bay 4 35 14 45°N Everett 46°N Taholah CANADA Seattle Nespelem 40 Aberdeen 44°N Wellpinit Browning Spokane 45°N Harlem Belcourt WAS HIN Box Wagner E GTO Plummer Elder IN N MA 10 Pablo E SUPER Wapato IO Poplar K R Toppenish A 43°N New L Town Fort Totten Red Lake NT 44°N O Lapwai RM Portland VE Sault MO Sainte Marie NTANA Cass Lake Siletz Pendleton 42°N K NH NORTH DAKOTA Ashland YOR EW 43°N Warm N Springs LA KE No H r Fort U t Yates Boston hw Billings R TS e Crow ET 41°N s Agency O S t HU Worcester O R N AC RE eg Lame Deer OTA NTARIO SS GON io MINNES E O MA 42°N n Sisseton K A Providence 23 Aberdeen L N I 39 Rochester R A Springfield Minneapolis 51 G Saint Paul T SIN I C WISCON Eagle H 40°N IDA Butte Buffalo Boise HO C I 6 41°N R M o E cky M SOUTH DAKOTA ou K AN ntai ICHIG n R A M egion Lower Brule Fort Thompson L E n Grand Rapids I io New York g 39°N e Milwaukee R Fort Hall R west 24 E d Detroit Mi E 40°N Fort Washakie K WYOMING LA Rosebud Pine Ridge Cleveland IA Redding Wagner AN Toledo LV 32 NSY PEN Philadelphia 38°N Chicago NJ A 39°N IOW Winnebago Pittsburgh Fort Wayne Elko 25 Great Plains Region Baltimore Des Moines MD E NEBRASKA OHIO D -

EMS P- *Treaties: Tribes
DOCUMENT RESUME 310 175 612 RC 011 603 TITLE Beyond Bows and Arrows. Resource Manual. INSTITUTION Civil Service Commission, Washington, D. C. Bureau of Intergovernaental Personaal Programs. PUB DATE Nay 79 NOTE 148p.; Prepared for the Dallas Region "Symposium on the American Indian" EMS P- E OF01 Plus Postage. PC Not Available from EDRS. DESCRIL 1S American History: *American Indian Culture: American Indian Education: *American Indians: Civil Rights: Education: Employment: Federal Government: *Federal Indian Relationship: *Government Role: Health: Housing: Population Trends: Reservations (Indian): *Treaties: Tribes IDENTIFIMIS *American Irlian History: Bureau of Indian Affairs: Cultural Contributions ABSTRACT In spite of their visible prominence and influence on almost emery aspect of our society, Aserican Indians remain theleast understood group of people. To acquaint symposium participantswith the American Indian and to produce greater understanding,this resource manual docuaents the historical treatmentand present status of Indians. Presented are: the constitutional status ofAmerican Indians, including soarces of federal power, tribalsovereignty, , powers of tribal self-government, hunting andfishing rights, domestic relations, taxation, legal statas of Indian individuals, constitutional immunity, the 1968 Indian Bill of Rights, rights and privileges of state citizenship, and wardship:American Indian tribes, Eskimo and Aleut groups for which the Bureau of Indian Affairs has responsibility: federal Indian policiesfrom the colonial period through the early 19701s: administrators of U.S. Federal Indian Policy: Cosmissioners of Indian Affairs from 1832 tothe present: important dates in federal Indian relationships:labor statistics: employment: education: health: relevance of Indianlife %* civilisation: housing: Indian population byregions: location of Adian lands and communities: and Indian tribes andorganization, including names and addresses of each leader. -
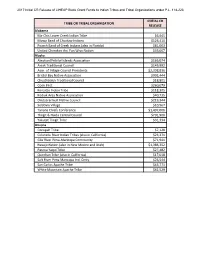
Initial CR Release of LIHEAP Block Grant Funds to Indian Tribes and Tribal Organizations Under P.L
2017 Initial CR Release of LIHEAP Block Grant Funds to Indian Tribes and Tribal Organizations under P.L. 114-223 INITIAL CR TRIBE OR TRIBAL ORGANIZATION RELEASE Alabama Ma-Chis Lower Creek Indian Tribe $6,945 Mowa Band of Choctaw Indians $126,410 Poarch Band of Creek Indians (also in Florida) $81,003 United Cherokee Ani-Yun Wiya Nation $33,007 Alaska Aleutian/Pribilof Islands Association $166,074 Aniak Traditional Council $149,992 Assn. of Village Council Presidents $2,398,836 Bristol Bay Native Association $902,444 Chuathbaluk Traditional Council $18,801 Cook Inlet $260,079 Kenaitze Indian Tribe $118,305 Kodiak Area Native Association $40,735 Orutsararmuit Native Council $219,344 Seldovia Village $10,967 Tanana Chiefs Conference $1,497,006 Tlingit & Haida Central Council $701,900 Yakutat Tlingit Tribe $31,334 Arizona Cocopah Tribe $7,128 Colorado River Indian Tribes (also in California) $23,271 Gila River Pima-Maricopa Community $71,941 Navajo Nation (also in New Mexico and Utah) $1,386,352 Pascua Yaqui Tribe $27,482 Quechan Tribe (also in California) $17,018 Salt River Pima Maricopa Ind. Cmty. $26,544 San Carlos Apache Tribe $43,771 White Mountain Apache Tribe $62,529 2017 Initial CR Release of LIHEAP Block Grant Funds to Indian Tribes and Tribal Organizations under P.L. 114-223 INITIAL CR TRIBE OR TRIBAL ORGANIZATION RELEASE California Berry Creek Rancheria $5,358 Big Valley Band of Pomo Indians $1,443 Bishop Paiute $20,194 Coyote Valley Pomo Band $4,451 Enterprise Rancheria $2,061 Hoopa Valley Tribe $36,927 Hopland Band $5,605 Karuk Tribe $26,789 Mooretown Rancheria $15,290 N. -

Indian Lands of Federally Recognized Tribes of the United States (1885
13 2° W 13 1° W 13 0° W 12 9° W 12 8° W 12 7° W 12 6° W 12 5° W 12 4° W 12 3° W 12 2° W 12 1° W 12 0° W 119 ° W 118 ° W 117 ° W 116 ° W 115 ° W 114 ° W 113 ° W 112 ° W 111 ° W 110 ° W 10 9° W 10 8° W 10 7° W 10 6° W 10 5° W 10 4° W 10 3° W 10 2° W 10 1° W 10 0° W 99 ° W 98 ° W 97 ° W 96 ° W 95 ° W 94 ° W 93 ° W 92 ° W 91 ° W 90 ° W 89 ° W 88 ° W 87 ° W 86 ° W 85 ° W 84 ° W 83 ° W 82 ° W 81 ° W 80 ° W 79 ° W 78 ° W 77 ° W 76 ° W 75 ° W 74 ° W 73 ° W 72 ° W 71 ° W 70 ° W 69 ° W 68 ° W 67 ° W 66 ° W 65 ° W 64 ° W 63 ° W 48 ° N 46 ° N 47 °N Neah Bay 4 35 14 45 °N Everett 46 °N Taholah CANADA Seattle Nespelem 40 Aberdeen 44 °N Wellpinit Browning Spokane 45 °N Harlem Be l court Box Wagner Plummer Elder 10 Pablo E SUPERI Wapato K O Poplar R Toppenish A 43 °N New L Town F o rt To t t e n Red Lake 44 °N Lapwai Portland Sault Sainte Marie Cass Lake Siletz Pendleton 42 °N Ashland 43 °N W arm Spri L ngs A KE No H r For t U t Ya tes Boston hw Billings R e Crow 41 °N s Age O t ncy Re N Worcester gio Lame Deer E ONTARIO 42 °N n Sis seton K A Providence 23 N L 39 A b e r d e e n Rochester A Springfield Minneapolis 51 G Saint Paul I E a ge l H 40 °N B utt e Boise C Buffalo I 6 41 °N R M ocky M E ountai K n R A egion Lower Bru le F o r t T ho m p s o n L E n Grand Rapids I io New York g 39 °N e Milwaukee R Fort Hall t R Midwes 24 Detroit E KE 40 °N Fort Washakie LA Rose bud Pine Ridg e Wa g ne r Cleveland Redding 32 Toledo Philadelphia 38 °N Chicago ATLANTIC OCEAN 39 °N Winnebago Pittsburgh Fort Wayne Elko 25 Gr e at P la i ns R eg io n Baltimore Des Moines -

Tribal Leaders Directory 2015
OKLAHOMA TRIBAL LEADERS DIRECTORY 2015 Governor Edwina Butler-Wolfe Chief Tarpie Yargee Chairman Lyman Guy Principal Chief Bill John Baker Chairperson Tammy Absentee Shawnee Tribe of Alabama Quassarte Tribal Town Apache Tribe of Oklahoma Francis-Fourkiller Cherokee Nation of Oklahoma Oklahoma Caddo Nation of Oklahoma 2025 South Gordon Cooper Drive P.O. Box 187 P.O. Box 1220 P.O. Box 487 P.O. Box 948 Shawnee, Oklahoma 74801 Wetumka, Oklahoma 74883 Anadarko, Oklahoma 73005 Binger, Oklahoma 73009 Tahlequah, Oklahoma 74465 [email protected] [email protected] [email protected] 405.275.4030 xt. 188 405.452.3987 405.247.9493 xt. 121 405.656.2344 918.456.0671 xt. 5283 Governor Eddie Hamilton Governor Bill Anoatubby Chief Gary Batton Chairman John A. “Rocky” Chairman Wallace Coffey Barrett Cheyenne & Arapaho Tribes of Chickasaw Nation Choctaw Nation of Oklahoma Citizen Potawatomi Nation Comanche Nation of Oklahoma Oklahoma 100 Red Moon Circle P.O. Box 1548 P.O. Box 1210 1601 South Gordon Cooper Drive P.O. Box 908 Concho, Oklahoma 73022 Ada, Oklahoma 74820 Durant, Oklahoma 74702 Shawnee, Oklahoma 74801 Lawton, Oklahoma 73502 [email protected] [email protected] [email protected] [email protected] [email protected] 405.422.7733 580.436.7280 580.924.8280 405.275.3121 580.492.4988 Acting President C.J. Watkins Chief Chester Brooks Chief Glenna J. Wallace Chairman Jeff Haozous Chairperson Gary Pratt Delaware Nation of Oklahoma Delaware Tribe of Oklahoma Eastern Shawnee Tribe of Fort Sill Apache Tribe of Iowa Tribe of Oklahoma Oklahoma Oklahoma P.O. -

Inventory of Flag in the Hall of Tribal Nations
Inventory of Flag in the Hall of Tribal Nations 1 Absentee-Shawnee Tribe of Indians of Oklahoma 2 Aroostook Band of Micmac Indians of Maine 3 Barona Group of Capitan Grande Band of Mission Indians of the Barona Reservation, California 4 Bay Mills Indian Community, Michigan 5 Blackfeet Tribe of the Blackfeet Indian Reservation of Montana 6 Bois Forte Band (Nett Lake), Minnesota Chippewa Tribe, Minnesota 7 Cabazon Band of Mission Indians, California 8 Caddo Nation of Oklahoma 9 Central Council of the Tlingit and Haida Indian Tribes 10 Chemehuevi Indian Tribe of the Chemehuevi Reservation, California 11 Cherokee Nation, Oklahoma 12 Chickasaw Nation, Oklahoma 13 Chippewa-Cree Indians of the Rocky Boy’s Reservation, Montana 14 Choctaw Nation of Oklahoma 15 Citizen Potawatomi Nation, Oklahoma 16 Colorado River Indian Tribes of the Colorado River Indian Reservation, Arizona and California 17 Confederated Salish & Kootenai Tribes of the Flathead Reservation, Montana 18 Confederated Tribes and Bands of the Yakama Nation, Washington 19 Confederated Tribes of Siletz Indians of Oregon (previously listed as the Confederated Tribes of the Siletz Reservation) 20 Confederated Tribes of the Coos, Lower Umpqua and Siuslaw Indians of Oregon 21 Confederated Tribes of the Grand Ronde Community of Oregon 22 Confederated Tribes of the Umatilla Reservation, Oregon 23 Confederated Tribes of the Warm Springs Reservation of Oregon 24 Cowlitz Indian Tribe, Washington 25 Crow Tribe of Montana, Apsaalooke Nation 26 Delaware Tribe of Indians, Oklahoma 27 Enterprise -

Oklahoma Indian Country Guide in This Edition of Newspapers in Education
he American Indian Cultural Center and Museum (AICCM) is honored Halito! Oklahoma has a unique history that differentiates it from any other Tto present, in partnership with Newspapers In Education at The Oklahoman, state in the nation. Nowhere else in the United States can a visitor hear first the Native American Heritage educational workbook. Workbooks focus on hand-accounts from 39 different American Indian Tribal Nations regarding the cultures, histories and governments of the American Indian tribes of their journey from ancestral homelands, or discover how Native peoples have Oklahoma. The workbooks are published twice a year, around November contributed and woven their identities into the fabric of contemporary Oklahoma. and April. Each workbook is organized into four core thematic areas: Origins, Oklahoma is deeply rooted in American Indian history and heritage. We hope Native Knowledge, Community and Governance. Because it is impossible you will use this guide to explore our great state and to learn about Okla- to cover every aspect of the topics featured in each edition, we hope the Humma. (“Red People” in the Choctaw language.)–Gena Timberman, Esq., workbooks will comprehensively introduce students to a variety of new subjects and ideas. We hope you will be inspired to research and find out more information with the help of your teachers and parents as well as through your own independent research. The American Indian Cultural Center and Museum would like to give special thanks to the Oklahoma Tourism & Recreation Department for generously permitting us to share information featured in the Oklahoma Indian Country Guide in this edition of Newspapers in Education. -

Central Plains Region
Research Guides for both historic and modern Native Communities relating to records held at the National Archives Arkansas Kansas Missouri Oklahoma Introduction Introduction Introduction Introduction Historic Native Communities Historic Native Communities Historic Native Communities Historic Native Communities Modern Native Communities Modern Native Communities Modern Native Communities Modern Native Communities Sample Document Delegates from 34 tribes in front of Creek Council House, Indian Joseph Matthews, Osage council member, author, historian, and Territory, 1880. National Archives. Rhodes Scholar, seated at home in front of his fireplace, https://catalog.archives.gov/id/519141 Oklahoma. December 16, 1937. National Archives. https://www.archives.gov/research/native- americans/pictures/select-list-082.html National Archives Native Communities Research Guides. https://www.archives.gov/education/native-communities Arkansas Native Communities There was a great deal movement of Native People from, to and across Arkansas in the early nineteenth century. Therefore, in order to perform a simple search of the GENERAL records of Arkansas’ Native People in the National Archives Online Catalog it is best to focus on National Park Service historic place applications, Osage and Quapaw records before 1824, and records of the Five Civilized Tribes as they traveled across Arkansas. Use the following search terms: Removal (Advanced Search, using Record Group 75) and “Arkansas Indian” (include quotation marks). The much broader search, Arkansas Indian, will have to be narrowed further by date range and document type. There are several great resources available for general information and material for kids about the Native People of Arkansas, such as the Native Languages and National Museum of the American Indian websites. -

TRIBAL GAMING COMPACT Between the MIAMI TRIBE of OKLAHOMA, the MODOC TRIBE of OKLAHOMA, and the STATE of OKLAHOMA
TRIBAL GAMING COMPACT Between the MIAMI TRIBE OF OKLAHOMA, THE MODOC TRIBE OF OKLAHOMA, and the STATE OF OKLAHOMA This Compact is made and entered into by and between the Miami Tribe of Oklahoma, a federally recognized Indian tribe, the Modoc Tribe of Oklahoma, a federally recognized Indian tribe (collectively or individually hereinafter referred to as "tribes"), and the State of Oklahoma ("state"), with respect to the operation of covered games (as defined herein) on the tribe's Indian lands as defined by the Indian Gaming Regulatory Act, 25 U.S.C., Section 2703(4). Part 1. TITLE This document shall be referred to as the "Miami Tribe of Oklahoma and Modoc Tribe of Oklahoma and State of Oklahoma Gaming Compact". Part 2. RECITALS 1. The tribes are federally recognized tribal governments possessing sovereign powers and rights of self-govemment. 2. The State of Oklahoma is a state of the United States of America possessing the sovereign powers and rights of a state. 3. The state and the tribes maintain a government-to-government relationship, and this Compact will help to foster mutual respect and understanding among Indians and non-Indians. 4. The United States Supreme Court has long recognized the right of an Indian tribe to regulate activity on lands within its jurisdiction. 5. The tribes desire to offer the play of covered games, as defined in paragraphs 5, 10, 1 1 and 12 of Part 3 of this Compact, as a means of generating revenues for purposes authorized by the Indian Gaming Regulatory Act, 25 U.S.C., Section 2701, et seq., including without limitation the support of tribal governmental programs, such as health care, housing, sewer and water projects, police, corrections, fire, judicial services, highway and bridge construction, general assistance for tribal elders, day care for the children, economic development, educational opportunities and other typical and valuable governmental services and programs for tribal members. -

OREGON LAWS 2019 SCR 12 SENATE CONCURRENT RESOLUTION 12 Whereas Kintpuash
OREGON LAWS 2019 SCR 12 SENATE CONCURRENT RESOLUTION 12 Whereas hostilities resumed and the Modocs were decisively defeated in the Battle of Dry Lake on May 10, 1873, scattering the surviving Modocs Whereas Kintpuash (“Strikes the Water into small groups; and Brashly”) was born in about 1837 along the Lost Whereas Kintpuash was captured with his family River in the ancestral territory of the Modoc tribe on June 4, 1873, effectively ending Modoc resistance; in southern Oregon; and and Whereas Kintpuash, also known as Captain Jack Whereas Kintpuash and five other Modoc warri- and Kientpoos, became a principal headman of the ors were tried by a federal military court, convicted Modocs by the early 1860s; and and sentenced to death; and Whereas Kintpuash was among the Modoc Whereas two of the Modocs had their sentences signatories to the 1864 treaty between the United reduced to life imprisonment, but Kintpuash, States and the Modoc, Klamath and Yahooskin Schonchin John, Black Jim and Boston Charley tribes; and were hanged at Fort Klamath on October 3, 1873, Whereas the 1864 treaty terms required the the first Indians to be tried and executed by the Modocs to relocate from their ancestral lands to the federal government for war crimes; and Whereas the remaining members of the Modoc Klamath Reservation; and tribe, numbering approximately 150, were herded Whereas Kintpuash and the Modocs initially into rail cars and sent as prisoners of war to the complied with the 1864 treaty but became disillu- Indian Territory (Oklahoma); and sioned with