09-002 Phrma Heartdis09
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endothelin System and Therapeutic Application of Endothelin Receptor
xperim ACCESS Freely available online & E en OPEN l ta a l ic P in h l a C r m f o a c l a o n l o r g u y o J Journal of ISSN: 2161-1459 Clinical & Experimental Pharmacology Research Article Endothelin System and Therapeutic Application of Endothelin Receptor Antagonists Abebe Basazn Mekuria, Zemene Demelash Kifle*, Mohammedbrhan Abdelwuhab Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia ABSTRACT Endothelin is a 21 amino acid molecule endogenous potent vasoconstrictor peptide. Endothelin is synthesized in vascular endothelial and smooth muscle cells, as well as in neural, renal, pulmonic, and inflammatory cells. It acts through a seven transmembrane endothelin receptor A (ETA) and endothelin receptor B (ETB) receptors belongs to G protein-coupled rhodopsin-type receptor superfamily. This peptide involved in pathogenesis of cardiovascular disorder like (heart failure, arterial hypertension, myocardial infraction and atherosclerosis), renal failure, pulmonary arterial hypertension and it also involved in pathogenesis of cancer. Potentially endothelin receptor antagonist helps the treatment of the above disorder. Currently, there are a lot of trails both per-clinical and clinical on endothelin antagonist for various cardiovascular, pulmonary and cancer disorder. Some are approved by FAD for the treatment. These agents are including both selective and non-selective endothelin receptor antagonist (ETA/B). Currently, Bosentan, Ambrisentan, and Macitentan approved -

BIOWORLD TODAY Inquiry
BIOWORLDTM TODAY THE DAILY BIOPHARMACEUTICAL NEWS SOURCE JUNE 30 , 2016 BIOTECH’S MOST RESPECTED NEWS SOURCE FOR MORE THAN 20 YEARS VOLUME 27, NO. 126 LIPID JAM NOT OVER EASILY STOPPED FOR FUTILITY Waffle house? FDA Galena Biopharma implodes as PRESENT review fidgets endpoint, puts cancer vaccine Neuvax future in doubt outcome details; By Jennifer Boggs, Managing Editor Esperion grilled on holdup With the bulk of Galena Biopharma Inc.’s value riding on cancer vaccine Neuvax By Randy Osborne, Staff Writer (nelipepimut-S), the firm’s shares predictably plunged to a new 52-week low Wednesday as an independent data monitoring committee (IDMC) recommended the Analysts had plenty of questions but PRESENT phase III study in breast cancer be stopped for futility following a planned Esperion Therapeutics Inc. offered few interim analysis. But it’s the troubling language in the IDMC’s letter, suggesting the answers regarding the FDA’s stalling on placebo arm might actually have bested the treatment arm, that could signal the end oral, once-daily bempedoic acid (ETC- of the road for Neuvax. 1002) for lipid lowering, after the agency See Galena, page 3 See Esperion, page 4 CHINA DEALS AND M&A IN THE CLINIC Pfizer invests in Asia Merck strikes cancer SUPER ‘NOVA’ with $350M biotech vaccines deal with Tesaro shares blast plant in Hangzhou Moderna, delivering off as niraparib hits By Haky Moon, Staff Writer $200M up front PFS in ovarian cancer HONG KONG – China’s economy may By Michael Fitzhugh, Staff Writer By Marie Powers, News Editor be slowing down, but multinationals Findings from the phase III NOVA trial are positioning themselves to leverage Cancer vaccines tailored to fit tumor- specific profiles are at the heart of a new of niraparib in women with recurrent it as best they can while navigating a ovarian cancer blasted shares of still-complex regulatory environment. -

Initiation of Francis Trial
August 25‚ 2008 ANTHERA PHARMACEUTICALS ADVANCES GLOBAL DEVELOPMENT STRATEGY FOR VARESPLADIB IN PATIENTS WITH ACUTE CORONARY SYNDROME WITH THE INITIATION OF FRANCIS TRIAL SAN MATEO, CA – August 25, 2008 – Anthera Pharmaceuticals Inc., a privately held biopharmaceutical company developing anti-inflammatory drugs, today announced the initiation of the FRANCIS (Fewer Recurrent Acute coronary events with Near-term Cardiovascular Inflammation Suppression) clinical trial designed to examine the impact of varespladib when administered to patients within 96 hours of an Acute Coronary Syndrome (ACS) event. The FRANCIS trial is designed to assess the impact of oral varespladib on known biological markers of cardiovascular risk. It will enroll up to 500 patients that will be treated for a minimum of six months. The study will be conducted at sites in North America and Europe. FRANCIS will provide insight into the prevention of secondary Major Adverse Cardiovascular Events (MACE) over the duration of the trial. In this study, MACE is defined as a composite endpoint consisting of cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, unstable angina, and a subset of revascularization following the initial event. During the course of the study, patients will receive therapeutic standard of care in addition to high dose Lipitor ® (atorvastatin). In previous clinical trials, varespladib, a potent and highly selective inhibitor of secretory phospholipase A 2 (sPLA 2), has demonstrated marked improvements in independent markers of cardiovascular risk including, a near complete suppression of the target enzyme sPLA 2, a clinically meaningful and statistically significant reduction in “bad” LDL cholesterol, and a reduction in C-reactive protein, a known marker of inflammation. -

Tetraphase Pharmaceuticals, Inc
WBB Securities, LLC Steve Brozak, DMH [email protected] (908) 518-7610 Tetraphase Pharmaceuticals, Inc. (NasdaqGS: TTPH) Initiating Coverage Initiating Coverage with a Speculative Buy Rating February 22, 2018 and a 12-Month Price Target of $6.00 Tetraphase Prepares for Commercialization of a Needed Antibiotic Tetraphase Pharmaceuticals, Inc. (TTPH) is Current Price $2.20 a clinical stage pharmaceutical company, 12 Month Target Price $6.00 with eravacycline as its lead candidate. It is a novel tetracycline-derived antibiotic to 12-Month Trading Range $2.05-$9.93 treat resistant and multidrug-resistant Market Capitalization (Mil) $113.50 infections, including multidrug-resistant Shares Outstanding (Mil) 51.59 Gram-negative infections. Following Avg. Daily Volume 872,642 successful IGNITE1 and IGNITE4 Phase 3 L.T. Debt (Mil) 0.00 trials in complicated intra-abdominal infections (cIAI), a New Drug Application Dividend/Yield N/A (NDA) was filed with the FDA and a Book Value P/S $2.92 Marketing Authorisation Application (MAA) was submitted to the EMA for IV NASDAQ Composite 7,218.23 eravacycline. S&P 500 2,701.33 Historical Performance and Disclosures on Page 10 - 11 Two days ago, TTPH announced an exclusive Source: QUODD+ licensing agreement with Everest Medicines Limited, a C-bridge Capital-backed biopharmaceutical company based in China, to develop and commercialize eravacycline in mainland China, Taiwan, Hong Kong, Macau, South Korea, and Singapore (known as the Territories). Tetraphase will receive an initial upfront payment of $7.0 million and may receive clinical and regulatory milestones of up to $16.5 million as well as a maximum of $20.0 million via achieving annual sales milestones. -

The Top 100 November, 2016 a List of Stocks Topping Our Custom 'Torpedo’ Screen
The Top 100 November, 2016 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. GPRO GoPro, Inc. Class A Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary UA Under Armour, Inc. Class A Consumer Discretionary CRC California Resources Corp Energy CRZO Carrizo Oil & Gas, Inc. Energy CWEI Clayton Williams Energy, Inc. Energy FANG Diamondback Energy, Inc. Energy GST Gastar Exploration, Inc. Energy MPLX MPLX LP Energy RSPP RSP Permian, Inc. Energy SLCA U.S. Silica Holdings, Inc. Energy AAC AAC Holdings, Inc. Health Care ABEO Abeona Therapeutics, Inc. Health Care ACAD ACADIA Pharmaceuticals Inc. Health Care ADMP Adamis Pharmaceuticals Corporation Health Care ADMS Adamas Pharmaceuticals, Inc. Health Care ADXS Advaxis, Inc. Health Care AIMT Aimmune Therapeutics Inc Health Care AKBA Akebia Therapeutics, Inc. Health Care ALDR Alder Biopharmaceuticals, Inc. Health Care ARDM Aradigm Corporation Health Care ARDX Ardelyx, Inc. Health Care ARLZ Aralez Pharmaceuticals Inc. Health Care ATRA Atara Biotherapeutics Inc Health Care BCRX BioCryst Pharmaceuticals, Inc. Health Care BLUE bluebird bio, Inc. Health Care CARA Cara Therapeutics Inc Health Care CDNA CareDx, Inc. Health Care CEMP Cempra, Inc. Health Care CERS Cerus Corporation Health Care CFMS ConforMIS Inc Health Care CLVS Clovis Oncology, Inc. Health Care COLL Collegium Pharmaceutical, Inc. Health Care CORI Corium International, Inc. Health Care CRMD CorMedix Inc. Health Care CSU Capital Senior Living Corporation Health Care DERM Dermira Inc Health Care DVAX Dynavax Technologies Corporation Health Care DXCM DexCom, Inc. Health Care EPZM Epizyme, Inc. Health Care FOLD Amicus Therapeutics, Inc. Health Care HRTX Heron Therapeutics Inc Health Care ICPT Intercept Pharmaceuticals, Inc. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

香港知識產權公報hong Kong Intellectual Property Journal
香港知識產權公報 Hong Kong Intellectual Property Journal 2007年7月27日 27 July 2007 公報編號 Journal No.: 225 公布日期 Publication Date: 27-07-2007 分項名稱 Section Name: 目錄 Contents * 目錄 Contents 根據專利條例第 20 條發表的指定專利申請記錄請求 Requests to Record Designated Patent Applications published under section 20 of the Patents Ordinance 1. 按國際專利分類排列 Arranged by International Patent Classification 2. 按發表編號排列 Arranged by Publication Number 3. 按申請編號排列 Arranged by Application Number 4. 按申請人姓名/名稱排列 Arranged by Name of Applicant 根據專利條例第 27 條發表批予標準專利 Granted Standard Patents published under section 27 of the Patents Ordinance 1. 按國際專利分類排列 Arranged by International Patent Classification 2. 按發表編號排列 Arranged by Publication Number 3. 按申請編號排列 Arranged by Application Number 4. 按專利所有人姓名/名稱排列 Arranged by Name of Proprietor 1/336 公報編號 Journal No.: 225 公布日期 Publication Date: 27-07-2007 分項名稱 Section Name: 目錄 Contents 根據專利條例第 118 條發表批予短期專利 Granted Short-term Patents published under section 118 of the Patents Ordinance 1. 按國際專利分類排列 Arranged by International Patent Classification 2. 按發表編號排列 Arranged by Publication Number 3. 按申請編號排列 Arranged by Application Number 4. 按專利所有人姓名/名稱排列 Arranged by Name of Proprietor 根據專利條例(第 514 章)公布的其他公告 Other Notices Published under the Patents Ordinance (Cap. 514) 根據專利條例第 20 條發表後撤回,當作已予撤回或被拒的申請 Applications Withdrawn, Deemed to have been Withdrawn, or Refused, after Publication under section 20 of the Patents Ordinance 根據專利條例第 39 條,標準專利因未繳續期費而停止有效 Standard Patents Ceased through Non-payment of Renewal Fees under section 39 of the Patents Ordinance 根據專利條例第 39(1)(b)條,標準專利 -

(12) United States Patent )-X- NZ
US008895596B2 (12) United States Patent (10) Patent No.: US 8,895,596 B2 Jiang et al. (45) Date of Patent: Nov. 25, 2014 (54) CYCLIC BENZMDAZOLE DERVATIVES (56) References Cited USEFUL AS ANT-DABETICAGENTS U.S. PATENT DOCUMENTS (75) Inventors: Jinlong Jiang, Scotch Plains, NJ (US); 5,596,025 A 1/1997 Oxman et al. Andrew J. Kassick, Scotch Plains, NJ 6,312,662 B1 11/2001 Erion et al. (US); Ahmet Kekec, Jersey City, NJ 6,489.476 B1 12/2002 Dang et al. (US); Iyassu K. Sebhat, Jersey City, NJ 7,098,220 B2 8, 2006 Rault et al. 7,268,145 B2 9, 2007 Matsumoto et al. (US) 2005, OO38068 A1 2/2005 Iyengar et al. 2005.0113283 A1 5/2005 Solow-Cordero et al. (73) Assignee: Merck Sharp & Dohme Corp, Rahway, 2005. O148643 A1 7/2005 Rui et al. NJ (US) 2005/O187277 A1 8/2005 Malti et al. 2005/0255415 A1 11/2005 Louwet et al. (*) Notice: Subject to any disclaimer, the term of this 2005/0272765 A1 12/2005 Feng et al. patent is extended or adjusted under 35 2006.0160872 A1 7/2006 Norman et al. 2006/0287356 Al 12/2006 Iyengaret al. U.S.C. 154(b) by 0 days. 2007, OO15665 A1 1/2007 Potluri et al. 2007/0O32529 A1 2/2007 Takagi et al. (21) Appl. No.: 13/578,302 2008. O1325O1 A1 6/2008 Sun et al. (22) PCT Fled: Feb. 21, 2011 FOREIGN PATENT DOCUMENTS (86) PCT NO.: PCT/US2011/025585 DE 33 16095 A1 11, 1983 EP O120403 A2 10, 1984 S371 (c)(1), EP O126030 A2 11, 1984 (2), (4) Date: Aug. -

February 11-12, 2013 the Waldorf Astoria New York
February 11-12, 2013 The Waldorf Astoria New York 15th ANNU AL EVENT Now in its fifteenth year, the BIO CEO & Investor Conference is the largest independent investor conference focused on leading publicly-traded biotech companies. The meeting provides a neutral forum where institutional investors, industry analysts, and senior biotechnology executives have the opportunity to shape the future investment landscape of the biotechnology industry. Reasons Top 10 to attend 1 Present your company story to an audience of targeted investors. 2 Hear the Washington perspective on the Affordable Care Act, debt ceiling, and other timely policy developments affecting the industry. 3 Evaluate fresh investment opportunities including compatible, complementary and competitive companies. 4 Learn about the hottest clinical developments and industry catalysts by attending the conference’s therapeutic workshops and business roundtables. 5 Attend fireside chats with CEOs who will share their recent company successes, what keeps the C-suite up at night, and where the industry’s leading companies are headed in 2013. 6 Gain access to BIO’s 1x1 Partnering System for scouting potential deal partners and optimizing your time at the event. 7 Access presentations from more than 140 established public and private biotech companies and non-profit funding organizations, including many you won’t hear from at other investor conferences. 8 Get the pulse on the current and proposed investment trends in biotechnology. 9 Network with peers, investors and potential partners attending the conference. 10 It’s the first NYC biotech conference of the year, kicking off a week of key industry events that you don’t want to miss. -
Example of Corresponding Structure of Excel Report, for E
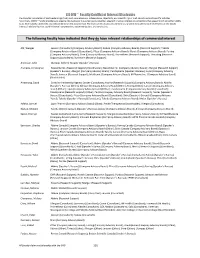
CDDW™ Faculty Conflict of Interest Disclosure The Canadian Association of Gastroenterology (CAG) must ensure balance, independence, objectivity, and scientific rigour in all educational and scientific activities. Accordingly, CDDW™ faculty members are expected to disclose to the audience any potential, apparent or actual interests or connections that appear to influence their ability to act with integrity, objectivity, and independence on the assigned task. The intent of this disclosure summary is to provide the audience with information on the faculty interests/relationships that could influence interpretations, recommendations, and conclusions. The following faculty have indicated that they do have relevant relationships of commercial interest Afif, Waqqas Janssen (Consultant) (Company Advisory Board); Abbvie (Company Advisory Board) (Research Support); Takeda (Company Advisory Board) (Consultant); Pfizer (Company Advisory Board); Merck (Company Advisory Board); Ferring (Company Advisory Board); Shire (Company Advisory Board); Prometheus (Research Support); Theradiag (Research Support) (Consultant); Buhlmann (Research Support) Anderson, John Olympus (Other); Norgine (Speaker's Bureau) Andrews, Christopher Newstrike Inc. (Research Support) (Stockholder); Newstrike Inc. (Company Advisory Board); Allergan (Research Support) (Speaker's Bureau); Allergan (Company Advisory Board); Pendopharm (Speaker's Bureau); Lupin (Company Advisory Board); Jannsen (Research Support); Medtronic (Company Advisory Board); M Pharma Inc. (Company Advisory Board) -

Form 13F-HR Filed 2019-02-14
SECURITIES AND EXCHANGE COMMISSION FORM 13F-HR Initial quarterly Form 13F holdings report filed by institutional managers Filing Date: 2019-02-14 | Period of Report: 2018-12-31 SEC Accession No. 0001567619-19-004367 (HTML Version on secdatabase.com) FILER DEERFIELD MANAGEMENT COMPANY, L.P. (SERIES C) Mailing Address Business Address 780 THIRD AVENUE, 37TH 780 THIRD AVENUE CIK:1009258| IRS No.: 000000000 | State of Incorp.:DE FLOOR 37TH FLOOR Type: 13F-HR | Act: 34 | File No.: 028-05366 | Film No.: 19606038 NEW YORK NY 10017 NEW YORK NY 10017 2125511600 Copyright © 2019 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document OMB APPROVAL UNITED STATES SECURITIES AND EXCHANGE OMB Number: 3235-0006 COMMISSION Expires: July 31, 2015 Washington, D.C. 20549 Estimated average burden hours per response: 23.8 FORM 13F FORM 13F COVER PAGE Report for the Calendar Year or Quarter Ended: 12-31-2018 Check here if Amendment: ☐ Amendment Number: This Amendment (Check only one.): ☐ is a restatement. ☐ adds new holdings entries. Institutional Investment Manager Filing this Report: Name: DEERFIELD MANAGEMENT COMPANY, L.P. (SERIES C) Address: 780 THIRD AVENUE, 37TH FLOOR NEW YORK, NY 10017 Form 13F File Number: 028-05366 The institutional investment manager filing this report and the person by whom it is signed hereby represent that the person signing the report is authorized to submit it, that all information contained herein is true, correct and complete, and that it is understood that all required items, statements, schedules, lists, and tables, are considered integral parts of this form. Person Signing this Report on Behalf of Reporting Manager: Name: James E. -

Board of Director Candidates
2021 Annual General Meeting Board of Director Candidates James I. Healy, M.D., Ph.D. (Current Board Member) James I. Healy, M.D., Ph.D. has served as a member of our board of directors since November 2014. Dr. Healy has been a General Partner of Sofinnova Investments, Inc. (formerly Sofinnova Ventures), a venture capital firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of Bolt Therapeutics, Inc. (Nasdaq: BOLT), Coherus BioSciences, Inc. (Nasdaq: CHRS), Karuna Therapeutics, Inc. (Nasdaq: KRTX), Natera, Inc. (Nasdaq: NTRA), NuCana plc (Nasdaq: NCNA), ObsEva SA (Nasdaq: OBSV) and Y-mAbs Therapeutics, Inc. (Nasdaq: YMAB) and two private companies. Previously, he served as a board member of Amarin Corporation, Auris Medical Holding AG, Edge Therapeutics, Inc., Hyperion Therapeutics, Inc., InterMune, Inc., Iterum Therapeutics PLC, Anthera Pharmaceuticals, Inc., Durata Therapeutics, Inc., CoTherix, Inc., Movetis NV and several private companies. Dr. Healy holds an M.D. and a Ph.D. in Immunology from Stanford University School of Medicine and holds a B.A. in Molecular Biology and a B.A. in Scandinavian Studies from the University of California, Berkeley. Jan Møller Mikkelsen (Current Board Member) Jan Møller Mikkelsen founded Ascendis Pharma and has served as President and Chief Executive Officer as well as Board member since December 2007 and currently serves on the board of Visen. From 2002 to 2006, Mr. Mikkelsen served as President and Chief Executive Officer of LifeCycle Pharma A/S, now Veloxis Pharmaceuticals A/S, which was a publicly traded biotechnology company.