Example of Corresponding Structure of Excel Report, for E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BIOWORLD TODAY Inquiry
BIOWORLDTM TODAY THE DAILY BIOPHARMACEUTICAL NEWS SOURCE JUNE 30 , 2016 BIOTECH’S MOST RESPECTED NEWS SOURCE FOR MORE THAN 20 YEARS VOLUME 27, NO. 126 LIPID JAM NOT OVER EASILY STOPPED FOR FUTILITY Waffle house? FDA Galena Biopharma implodes as PRESENT review fidgets endpoint, puts cancer vaccine Neuvax future in doubt outcome details; By Jennifer Boggs, Managing Editor Esperion grilled on holdup With the bulk of Galena Biopharma Inc.’s value riding on cancer vaccine Neuvax By Randy Osborne, Staff Writer (nelipepimut-S), the firm’s shares predictably plunged to a new 52-week low Wednesday as an independent data monitoring committee (IDMC) recommended the Analysts had plenty of questions but PRESENT phase III study in breast cancer be stopped for futility following a planned Esperion Therapeutics Inc. offered few interim analysis. But it’s the troubling language in the IDMC’s letter, suggesting the answers regarding the FDA’s stalling on placebo arm might actually have bested the treatment arm, that could signal the end oral, once-daily bempedoic acid (ETC- of the road for Neuvax. 1002) for lipid lowering, after the agency See Galena, page 3 See Esperion, page 4 CHINA DEALS AND M&A IN THE CLINIC Pfizer invests in Asia Merck strikes cancer SUPER ‘NOVA’ with $350M biotech vaccines deal with Tesaro shares blast plant in Hangzhou Moderna, delivering off as niraparib hits By Haky Moon, Staff Writer $200M up front PFS in ovarian cancer HONG KONG – China’s economy may By Michael Fitzhugh, Staff Writer By Marie Powers, News Editor be slowing down, but multinationals Findings from the phase III NOVA trial are positioning themselves to leverage Cancer vaccines tailored to fit tumor- specific profiles are at the heart of a new of niraparib in women with recurrent it as best they can while navigating a ovarian cancer blasted shares of still-complex regulatory environment. -

Initiation of Francis Trial
August 25‚ 2008 ANTHERA PHARMACEUTICALS ADVANCES GLOBAL DEVELOPMENT STRATEGY FOR VARESPLADIB IN PATIENTS WITH ACUTE CORONARY SYNDROME WITH THE INITIATION OF FRANCIS TRIAL SAN MATEO, CA – August 25, 2008 – Anthera Pharmaceuticals Inc., a privately held biopharmaceutical company developing anti-inflammatory drugs, today announced the initiation of the FRANCIS (Fewer Recurrent Acute coronary events with Near-term Cardiovascular Inflammation Suppression) clinical trial designed to examine the impact of varespladib when administered to patients within 96 hours of an Acute Coronary Syndrome (ACS) event. The FRANCIS trial is designed to assess the impact of oral varespladib on known biological markers of cardiovascular risk. It will enroll up to 500 patients that will be treated for a minimum of six months. The study will be conducted at sites in North America and Europe. FRANCIS will provide insight into the prevention of secondary Major Adverse Cardiovascular Events (MACE) over the duration of the trial. In this study, MACE is defined as a composite endpoint consisting of cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, unstable angina, and a subset of revascularization following the initial event. During the course of the study, patients will receive therapeutic standard of care in addition to high dose Lipitor ® (atorvastatin). In previous clinical trials, varespladib, a potent and highly selective inhibitor of secretory phospholipase A 2 (sPLA 2), has demonstrated marked improvements in independent markers of cardiovascular risk including, a near complete suppression of the target enzyme sPLA 2, a clinically meaningful and statistically significant reduction in “bad” LDL cholesterol, and a reduction in C-reactive protein, a known marker of inflammation. -

February 11-12, 2013 the Waldorf Astoria New York
February 11-12, 2013 The Waldorf Astoria New York 15th ANNU AL EVENT Now in its fifteenth year, the BIO CEO & Investor Conference is the largest independent investor conference focused on leading publicly-traded biotech companies. The meeting provides a neutral forum where institutional investors, industry analysts, and senior biotechnology executives have the opportunity to shape the future investment landscape of the biotechnology industry. Reasons Top 10 to attend 1 Present your company story to an audience of targeted investors. 2 Hear the Washington perspective on the Affordable Care Act, debt ceiling, and other timely policy developments affecting the industry. 3 Evaluate fresh investment opportunities including compatible, complementary and competitive companies. 4 Learn about the hottest clinical developments and industry catalysts by attending the conference’s therapeutic workshops and business roundtables. 5 Attend fireside chats with CEOs who will share their recent company successes, what keeps the C-suite up at night, and where the industry’s leading companies are headed in 2013. 6 Gain access to BIO’s 1x1 Partnering System for scouting potential deal partners and optimizing your time at the event. 7 Access presentations from more than 140 established public and private biotech companies and non-profit funding organizations, including many you won’t hear from at other investor conferences. 8 Get the pulse on the current and proposed investment trends in biotechnology. 9 Network with peers, investors and potential partners attending the conference. 10 It’s the first NYC biotech conference of the year, kicking off a week of key industry events that you don’t want to miss. -

Board of Director Candidates
2021 Annual General Meeting Board of Director Candidates James I. Healy, M.D., Ph.D. (Current Board Member) James I. Healy, M.D., Ph.D. has served as a member of our board of directors since November 2014. Dr. Healy has been a General Partner of Sofinnova Investments, Inc. (formerly Sofinnova Ventures), a venture capital firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of Bolt Therapeutics, Inc. (Nasdaq: BOLT), Coherus BioSciences, Inc. (Nasdaq: CHRS), Karuna Therapeutics, Inc. (Nasdaq: KRTX), Natera, Inc. (Nasdaq: NTRA), NuCana plc (Nasdaq: NCNA), ObsEva SA (Nasdaq: OBSV) and Y-mAbs Therapeutics, Inc. (Nasdaq: YMAB) and two private companies. Previously, he served as a board member of Amarin Corporation, Auris Medical Holding AG, Edge Therapeutics, Inc., Hyperion Therapeutics, Inc., InterMune, Inc., Iterum Therapeutics PLC, Anthera Pharmaceuticals, Inc., Durata Therapeutics, Inc., CoTherix, Inc., Movetis NV and several private companies. Dr. Healy holds an M.D. and a Ph.D. in Immunology from Stanford University School of Medicine and holds a B.A. in Molecular Biology and a B.A. in Scandinavian Studies from the University of California, Berkeley. Jan Møller Mikkelsen (Current Board Member) Jan Møller Mikkelsen founded Ascendis Pharma and has served as President and Chief Executive Officer as well as Board member since December 2007 and currently serves on the board of Visen. From 2002 to 2006, Mr. Mikkelsen served as President and Chief Executive Officer of LifeCycle Pharma A/S, now Veloxis Pharmaceuticals A/S, which was a publicly traded biotechnology company. -

AVEO PHARMACEUTICALS, INC. (Name of Registrant As Specified in Its Charter)
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 14A (Rule 14a-101) INFORMATION REQUIRED IN PROXY STATEMENT SCHEDULE 14A INFORMATION Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934 Filed by the Registrant x Filed by a party other than the Registrant ¨ Check the appropriate box: ¨ Preliminary Proxy Statement ¨ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) x Definitive Proxy Statement ¨ Definitive Additional Materials ¨ Soliciting Material Pursuant to §240.14a-12 AVEO PHARMACEUTICALS, INC. (Name of Registrant as Specified In Its Charter) (Name of Person(s) Filing Proxy Statement, if Other Than The Registrant) Payment of Filing Fee (Check the appropriate box): x No fee required. ¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: ¨ Fee paid previously with preliminary materials: ¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. -

Guidelines with Regard to the Composition, Calculation and Management of the Index
INDEX METHODOLOGY Solactive Pharma Breakthrough Value Index Version 2.1 dated September 03, 2020 Contents Important Information 1. Index specifications 1.1 Short Name and ISIN 1.2 Initial Value 1.3 Distribution 1.4 Prices and Calculation Frequency 1.5 Weighting 1.6 Index Committee 1.7 Publication 1.8 Historical Data 1.9 Licensing 2. Composition of the Index 2.1 Selection of the Index Components 2.2 Ordinary Adjustment 2.3 Extraordinary Adjustment 3. Calculation of the Index 3.1 Index Formula 3.2 Accuracy 3.3 Adjustments 3.4 Dividends and other Distributions 3.5 Corporate Actions 3.6 Correction Policy 3.7 Market Disruption 3.8 Consequences of an Extraordinary Event 4. Definitions 5. Appendix 5.1 Contact Details 5.2 Calculation of the Index – Change in Calculation Method 2 Important Information This document (“Index Methodology Document”) contains the underlying principles and regulations regarding the structure and the operating of the Solactive Pharma Breakthrough Value Index. Solactive AG shall make every effort to implement regulations. Solactive AG does not offer any explicit or tacit guarantee or assurance, neither pertaining to the results from the use of the Index nor the Index value at any certain point in time nor in any other respect. The Index is merely calculated and published by Solactive AG and it strives to the best of its ability to ensure the correctness of the calculation. There is no obligation for Solactive AG – irrespective of possible obligations to issuers – to advise third parties, including investors and/or financial intermediaries, of any errors in the Index. -

The Weekly Shot Biotech Issue a Weekly Summary of Healthcare Industry Valuation and Near-Term Catalysts June 17, 2010
Small Cap The Weekly Shot Biotech Issue June 17, 2010 A weekly summary of healthcare industry valuation and near-term catalysts The Weekly Shot: Overview and Comment - Small Cap Biotechnology Next week's sector highlights include LGND’s Thursday analyst event at the Eventi - Pharmaceuticals and Large Cap Biotech Hotel in NYC. The company on 6/15 announced updated 2010 revenue guidance of approx $25M, op ex of approx $30M, and expects to finish the year with $30M - Generics and Specialty Pharmaceuticals in cash (vs approx $43M as of 1Q10). Management will likely focus on partner GSK’s progress with add’l trials of Promacta (for ITP), which could potentially expand the drug’s label to Hep C, AML, and MDS (LGND receives <10% royalty from GSK). Investors should focus on pipeline plans following LGND’s opportunistic 2008/09 M&A activity. Key pipeline programs include LGD-4033 (ph.I, SARM candidate from PCOP) and RG7348, partnered with Roche (ph.I, Hep C candidate from MBRX). We do not expect major data announcements at the event. FDA’s Pediatric Drugs Advisory Committee will meet Monday to discuss pediatric safety reviews of multiple approved drugs, including Kogenate, Casodex, Apidra, NovoLog, Arimidex, Desmopressin, Prevacid, Nexium, Aciphex, Priolex, OraVerse, Zemuron, and Suprane . While important from a public safety perspective, we do not anticipate regulatory activity to be announced. Brian Lian, Ph.D. Small caps biotechs rebounded mid-week as elevated volatility continued across 212.500.6646 [email protected] the broader market. Investors are struggling to balance economic data supporting a modest recovery against concerns on EU debt loads, financial reform legislation, and aggressive govt rhetoric on BP’s oil spill. -

09-002 Phrma Heartdis09
201R1e port M EDICINES IN D EVELOPMENT FOR Heart Disease and Stroke PRESENTED BY AMERICA ’ S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Companies Are Developing Nearly 300 Medicines for Cardiovascular Disease iopharmaceutical research companies are MEDICINES IN DEVELOPMENT FOR HEART DISEASE AND STROKE * developing 299 new medicines for two of the Bleading causes of death of Americans—heart disease Acute Cor onary Syndrome 22 and stroke. The work continues the momentum of drug Adjunctive Therapies 5 Arrhythmia/Atrial Fibrillation 15 discovery that has helped cut deaths from these diseases Atherosclerosis 15 by 28 percent between 1997 and 2007. All of the medicines Coronary Artery Disease 9 are either in clinical trials or awaiting review by the Heart Attack 17 Food and Drug Administration. Heart Failure 36 Hypertension 27 According to the National Center for Health Statistics, Imaging Agents 11 heart disease has topped the list of killer diseases every Ischemic Disorders 23 43 year but one since 1900. (The exception was 1918, Lipid Disorders Peripheral Vascular Disease 20 when an influenza epidemic killed more than 450,000 Pulmonary Vascular Disease 17 Americans.) Stroke 27 Thrombosis 28 Thanks in large part to new drug treatments, death rates Other 35 from heart disease and stroke are falling. In 2008, stroke dropped to the fourth leading cause of death after being *S ome medicines are listed in more than one category. the third for over 50 years. According to the National Heart, Lung and Blood Institute (NHLBI), if death rates The medicines in development include 43 for lipid were the same as those of 30 years ago, 815,000 more disorders, such as high cholesterol, 36 for heart failure, Americans would die of heart disease annually and 27 for high blood pressure, 17 for heart attacks, and 250,000 more would die of stroke. -

The Bottom 99
The Top 100 March, 2016 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. CHTR Charter Communications, Inc. Class A Consumer Discretionary GTIM Good Times Restaurants Inc. Consumer Discretionary HEAR Turtle Beach Corporation Consumer Discretionary LGF Lions Gate Entertainment Corp. Consumer Discretionary NFLX Netflix, Inc. Consumer Discretionary RLOC ReachLocal, Inc. Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary RKDA Arcadia Biosciences, Inc. Consumer Staples CLR Continental Resources, Inc. Energy CRZO Carrizo Oil & Gas, Inc. Energy CXO Concho Resources Inc. Energy ERN Erin Energy Corporation Energy FANG Diamondback Energy, Inc. Energy GST Gastar Exploration, Inc. Energy LNG Cheniere Energy, Inc. Energy LPI Laredo Petroleum, Inc. Energy MCF Contango Oil & Gas Company Energy RSPP RSP Permian, Inc. Energy WLL Whiting Petroleum Corporation Energy WTI W&T Offshore, Inc. Energy XEC Cimarex Energy Co. Energy ABEO Abeona Therapeutics, Inc. Health Care ACAD ACADIA Pharmaceuticals Inc. Health Care ADXS Advaxis, Inc. Health Care AGEN Agenus Inc. Health Care AGIO Agios Pharmaceuticals, Inc. Health Care ALDR Alder Biopharmaceuticals, Inc. Health Care ALNY Alnylam Pharmaceuticals, Inc Health Care ALXN Alexion Pharmaceuticals, Inc. Health Care ANTH Anthera Pharmaceuticals, Inc. Health Care ARGS Argos Therapeutics, Inc. Health Care ATRA Atara Biotherapeutics Inc Health Care ATRC AtriCure, Inc. Health Care AVGR Avinger, Inc. Health Care BDSI BioDelivery Sciences International, Inc. Health Care BLCM Bellicum Pharmaceuticals Inc Health Care BLUE bluebird bio, Inc. Health Care CERS Cerus Corporation Health Care CLVS Clovis Oncology, Inc. Health Care CORI Corium International, Inc. Health Care CPHD Cepheid Health Care CRIS Curis, Inc. Health Care CRMD CorMedix Inc. Health Care CTIC CTI BioPharma Corp. -
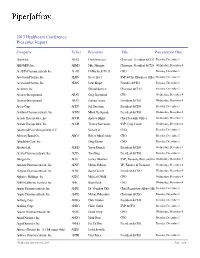
Presenter Report
2013 Healthcare Conference Presenter Report Company Ticker Presenter Title Presentation Date Abaxis Inc. ABAX Clint Severson Chairman, President & CEO Tuesday, December 3 ABIOMED, Inc. ABMD Mike Minogue Chairman, President & CEO Wednesday, December 4 ACADIA Pharmaceuticals Inc. ACAD Uli Hacksell, Ph.D. CEO Tuesday, December 3 Acceleron Pharma, Inc. XLRN Steve Ertel SVP & Chief Business Office Tuesday, December 3 Acceleron Pharma, Inc. XLRN John Knopf Founder & CEO Tuesday, December 3 Accellent, Inc. Donald Spence Chairman & CEO Tuesday, December 3 Accuray Incorporated ARAY Greg Lichtwardt CFO Wednesday, December 4 Accuray Incorporated ARAY Joshua Levine President & CEO Wednesday, December 4 Aceto Corp. ACET Sal Guccione President & CEO Tuesday, December 3 Achillion Pharmaceuticals, Inc. ACHN Milind Deshpande President & CEO Wednesday, December 4 Acorda Therapeutics, Inc. ACOR Andrew Blight Chief Scientific Officer Wednesday, December 4 Acorda Therapeutics, Inc. ACOR Tierney Saccavino SVP, Corp Comm Wednesday, December 4 Advanced Pain Management, S.C. Vishal Lal CEO Tuesday, December 3 Advisory Board Co. ABCO Robert Musslewhite CEO Tuesday, December 3 Affordable Care, Inc. Doug Brown CEO Tuesday, December 3 Alcobra Ltd. ADHD Yaron Daniely President & CEO Wednesday, December 4 Alexza Pharmaceuticals, Inc. ALXA Tom King President & CEO Tuesday, December 3 Allergan Inc. AGN James Hindman SVP, Treasury, Risk, and Inv Wednesday, December 4 Alnylam Pharmaceuticals, Inc. ALNY Michael Mason VP, Finance & Treasurer Wednesday, December 4 Alnylam Pharmaceuticals, Inc. ALNY Barry Greene President & COO Wednesday, December 4 Alphatec Holdings, Inc. ATEC Micheal O'Neill CFO Wednesday, December 4 AMN Healthcare Services Inc. AHS Brian Scott CFO Wednesday, December 4 Ampio Pharmaceuticals, Inc. AMPE Dr. Vaughan Clift Chief Regulatory Affairs Offic Tuesday, December 3 Ampio Pharmaceuticals, Inc. -

Program, Abstracts & Conference Information
Program, Abstracts & Conference Information 19 – 24 JUNE 2016 | FAIRMONT SOUTHAMPTON | BERMUDA We wish to thank our corporate sponsors for their generous support Recovery of Biological Products XVII FAIRMONT SOUTHAMPTON On-Demand Affinity Purification Solutions BERMUDA 19 – 24 JUNE 2016 We wish to thank our corporate sponsors for their generous support Recovery of Biological Products XVII FAIRMONT SOUTHAMPTON BERMUDA 19 – 24 JUNE 2016 AN INTERNATIONAL CONFERENCE Sponsored by: The American Chemical Society Division of Biochemical Technology Conference Management Provided by: Precision Meetings & Events 301 N. Fairfax St., Suite 104 Alexandria, VA 22314 USA Table of Contents Conference & Session Chairs . 3 Welcome Letter . 4 General Conference Information . 5 Activity Information . 7 Program Overview . 10 Daily Schedule . 12 Sunday . 12 Monday . 12 Tuesday . 13 Wednesday . 13 Thursday . 14 Friday . 14 Keynote Speakers . 15 Oral Abstracts . 21 The Origin of Impurities . 21 New Materials for Downstream Bioprocessing . 23 The Marriage of High Throughput Screening and Modeling . 26 Alternative Expression Systems: Strategic Impact for Biopharmaceuticals? . 28 Priorities in Cost and Performance Improvements . 30 Purification of Non-Protein Therapeutics . 32 Purification – from Platform to Diversity . 35 Process Challenges with Biosimilars . 38 Next Generation Unit Operations & Integrated Processes . 40 Biomolecular Modeling for Manufacturability . 43 New Developments in PAT and QbD . 45 Increasing Patient Access to Biopharmaceuticals . 47 Debates -

February 10-11, 2014 the Waldorf Astoria New York
February 10-11, 2014 The Waldorf Astoria New York Now in its sixteenth year, the BIO CEO & Investor Conference is the largest independent investor conference focused on leading publicly-traded biotech companies, with a special focus on select established private companies. The meeting provides a neutral forum where institutional investors, industry analysts, and senior biotechnology executives have the opportunity to shape the future investment landscape of the biotechnology industry. Reasons Top 10 to attend 1 35% of the biotechs who filed an IPO in 2013 will be presenting at this year’s BIO CEO & Investor Conference. 2 Hear the Washington perspective on the Affordable Care Act and other timely policy developments affecting the industry. 3 Evaluate fresh investment opportunities including compatible, complementary and competitive companies. 4 Learn about the hottest clinical developments and industry catalysts by attending the conference’s therapeutic workshops and business roundtables. 5 Attend fireside chats with CEOs who will share their recent company successes, what keeps the C-suite up at night, and where the industry’s leading companies are headed in 2014. 6 Gain access to BIO’s 1x1 Partnering SystemTM for scouting potential investments and deal partners, optimizing your time at the event. 7 Hear presentations from more than 150 established public and private biotech companies and non-profit funding organizations, including many you won’t hear from at other investor conferences. 8 Get the pulse of the current and proposed investment