The Impact of Land Use on Invertebrate Assemblages in the Succulent Karoo, South Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 1: General Introduction
THE CHEMOTAXONOMY, PHYLOGENY AND BIOLOGICAL ACTIVITY OF THE GENUS ERIOCEPHALUS L. (ASTERACEAE) Elizabeth Wanjiku Njenga A thesis submitted to the Faculty of Health Sciences, University of the Witwatersrand, in fulfilment of the requirements for the Degree Of Doctor of Philosophy Johannesburg, 2005. i DECLARATION I declare that this thesis is my own work. It is submitted for the degree of Doctor of Philosophy in the University of the Witwatersrand, Johannesburg. It has not been submitted for any degree or examination at any other university. The abstracts and copies of paper(s) included are part of this work. Signature Date ii DEDICATION To Joy, Shalom and George, my lifetime friends, for their love, courage, strength and prayers that inspired me to face all the challenges… iii ABSTRACT The genus Eriocephalus commonly known as ‘wild rosemary’, ‘Cape snow bush’, or ‘kapokbos’ is a member of the family Asteraceae (tribe Anthemideae). The genus is endemic to southern Africa, with the highest concentration of species in the Western and Northern Cape. The genus comprises 32 species and a total of 42 taxa, which are distributed in South Africa, Namibia, Botswana, and Lesotho. The characters used in species delimitation are purely based on morphological variation in floral and foliar parts and are highly homoplastic due to phenotypic plasticity. In many cases these features are not sufficiently distinctive, as some taxa tend to exhibit dimorphism in some character states such as the presence of opposite and alternate leaves. In some species there is extensive intergrading of the major diagnostic characters leading to uncertainty in species delimitation. -

Genetic Diversity and Evolution in Lactuca L. (Asteraceae)
Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis committee Promotor Prof. Dr M.E. Schranz Professor of Biosystematics Wageningen University Other members Prof. Dr P.C. Struik, Wageningen University Dr N. Kilian, Free University of Berlin, Germany Dr R. van Treuren, Wageningen University Dr M.J.W. Jeuken, Wageningen University This research was conducted under the auspices of the Graduate School of Experimental Plant Sciences. Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr A.P.J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Monday 25 January 2016 at 1.30 p.m. in the Aula. Zhen Wei Genetic diversity and evolution in Lactuca L. (Asteraceae) - from phylogeny to molecular breeding, 210 pages. PhD thesis, Wageningen University, Wageningen, NL (2016) With references, with summary in Dutch and English ISBN 978-94-6257-614-8 Contents Chapter 1 General introduction 7 Chapter 2 Phylogenetic relationships within Lactuca L. (Asteraceae), including African species, based on chloroplast DNA sequence comparisons* 31 Chapter 3 Phylogenetic analysis of Lactuca L. and closely related genera (Asteraceae), using complete chloroplast genomes and nuclear rDNA sequences 99 Chapter 4 A mixed model QTL analysis for salt tolerance in -

Monographs of Invasive Plants in Europe: Carpobrotus Josefina G
Monographs of invasive plants in Europe: Carpobrotus Josefina G. Campoy, Alicia T. R. Acosta, Laurence Affre, R Barreiro, Giuseppe Brundu, Elise Buisson, L Gonzalez, Margarita Lema, Ana Novoa, R Retuerto, et al. To cite this version: Josefina G. Campoy, Alicia T. R. Acosta, Laurence Affre, R Barreiro, Giuseppe Brundu, etal.. Monographs of invasive plants in Europe: Carpobrotus. Botany Letters, Taylor & Francis, 2018, 165 (3-4), pp.440-475. 10.1080/23818107.2018.1487884. hal-01927850 HAL Id: hal-01927850 https://hal.archives-ouvertes.fr/hal-01927850 Submitted on 11 Apr 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ARTICLE Monographs of invasive plants in Europe: Carpobrotus Josefina G. Campoy a, Alicia T. R. Acostab, Laurence Affrec, Rodolfo Barreirod, Giuseppe Brundue, Elise Buissonf, Luís Gonzálezg, Margarita Lemaa, Ana Novoah,i,j, Rubén Retuerto a, Sergio R. Roiload and Jaime Fagúndez d aDepartment of Functional Biology, Area of Ecology, Faculty of Biology, Universidade de Santiago de Compostela, Santiago de Compostela, Spain; bDipartimento -

In Vitro Culture and Studying the Chemical Composition of the Essential Oils Extracted from Three Samples of Eriocephalus Africanus L
Scientific J. Flowers & Ornamental Plants www.ssfop.com/journal ISSN: 2356-7864 doi: 10.21608/sjfop.2018.24212 IN VITRO CULTURE AND STUDYING THE CHEMICAL COMPOSITION OF THE ESSENTIAL OILS EXTRACTED FROM THREE SAMPLES OF ERIOCEPHALUS AFRICANUS L. PLANT IN EGYPT T.A.D. Mohamed*, A.M.A. Habib*, M.M. EL-Zefzafy** and A.I.E. Soliman** *Ornamental Horticulture Dept., Fac. Agric., Cairo Univ., Egypt. ** Medicinal Plants (Plant Tissue Culture) Dept., National Organization for Drug Control and Research (NODCAR), Giza, Egypt. ABSTRACT: The present study aimed to establish new protocol for propagation via tissue culture techniques to observe the effect of plant growth regulators especially cytokinins, gibberellic acid and auxins with different concentrations on in vitro growth of Eriocephalus africanus L. for improving the potentiality of regeneration and secondary metabolites production and identification of the main active constituents of volatile oil by GC/MS. The results showed that, the best sterilization treatment was the shoot tip explants rinsed in a solution of clorox at 15% for 15 min was gave the highest values for survival percentage and plant strength 100% and 4.58, respectively also B5 medium at full strength gave the best results in the both growth measurements. BAP at 2.00 mg/l recorded the highest values in survival percentage (93.33%), shootlet number/cluster (16.50) and shootlet strength (4.50), respectively. Using the high level from GA3 (4.00 mg/l) in medium was more effective in the elongation of shootlets. In rooting stage B5 medium supplemented with 0.50 mg/l Scientific J. Flowers & IBA and 0.15% active charcoal was more effective for increasing root Ornamental Plants, number/explant to 8.67 and root length to 5.78 cm. -

La Tribu Anthemideae Cass. (Asteraceae) En La Flora Alóctona De La Península Ibérica E Islas Baleares (Citas Bibliográficas Y Aspectos Etnobotánicos E Históricos)
Monografías de la Revista Bouteloua 9 La tribu Anthemideae Cass. (Asteraceae) en la flora alóctona de la Península Ibérica e Islas Baleares (Citas bibliográficas y aspectos etnobotánicos e históricos) DANIEL GUILLOT ORTIZ Abril de2010 Fundación Oroibérico & Jolube Consultor Editor Ambiental La tribu Anthemideae en la flora alóctona de la Península Ibérica e Islas Baleares Agradecimientos: A Carles Benedí González, por sus importantes aportaciones y consejos en el desarrollo de este trabajo. La tribu Anthemideae Cass. (Asteracea e) en la flora alóctona de la Península Ibérica e Islas Baleares (Citas bibliográficas y aspectos etnobotánicos e históricos) Autor: Daniel GUILLOT ORTIZ Monografías de la revista Bouteloua, nº 9, 158 pp. Disponible en: www.floramontiberica.org [email protected] En portada, Tanacetum parthenium, imagen tomada de la obra Köhler´s medicinal-Pflanzen, de Köhler (1883-1914). En contraportada, Anthemis austriaca, imagen tomada de la obra de Jacquin (1773-78) Floræ Austriacæ. Edición ebook: José Luis Benito Alonso (Jolube Consultor y Editor Ambiental. www.jolube.es) Jaca (Huesca), y Fundación Oroibérico, Albarracín (Teruel). Abril de 2010. ISBN ebook: 978-84-937811-0-1 Derechos de copia y reproducción gestionados po r el Centro Español de Derechos Reprográficos. Monografías Bouteloua, nº 9 2 ISBN: 978-84-937811-0-1 La tribu Anthemideae en la flora alóctona de la Península Ibérica e Islas Baleares INTRODUCCIÓN Incluimos en este trabajo todos los taxones citados como alóctonos de la tribu Anthemideae en la Península Ibérica e Islas Baleares en obras botánicas, tanto actuales como de los siglos XVIII-XIX y principios del siglo XX. Para cada género representado, incluimos información sobre aspectos como la etimología, sinonimia, descripción, número de especies y corología. -

Are Plants Used for Skin Care in South Africa Fully Explored?
Are plants used for skin care in South Africa fully explored? Namrita Lall*, Navneet Kishore Department of Plant Science, Plant Science Complex, University of Pretoria, Pretoria-0002, South Africa Corresponding Author: Prof. Namrita Lall Department of Plant Science, Plant Sciences Complex, University of Pretoria, Pretoria-0002, South Africa E-mail: [email protected] Phone: 012-420-2524; Fax: +27-12-420-6668 Co-author: Dr. Navneet Kishore Department of Plant Science, Plant Sciences Complex, University of Pretoria, Pretoria-0002, South Africa E-mail: [email protected] Phone: +27-12-420-6995 Graphical Abstract Scientific validation of South African medicinal plants used traditionally for skin care and their pharmacological properties associated with treating skin conditions. 1 Contents 1. Introduction 2. Plant as natural source for skin care 3. Abundance of active constituents is the backbone of phyto-derived products 4. Plants grown in South Africa, a potential source for new preparation with beneficial effects on the skin 4.1. Aloe ferox Mill. 4.2. Aspalathus linearis (Burm.f.) R. Dahlgren 4.3. Calodendrum capense (L.f.) Thunb. 4.4. Citrullus lanatus (Thunb.) 4.5. Elaeis guineensis Jacq 4.6. Eriocephalus africanus L. 4.7. Eriocephalus punctulatus L. 4.8. Greyia flanaganii Bolus 4.9. Olea europaea L. subsp. africana (Mill.) P.S.Green 4.10. Pelargonium graveolens L'Her. 4.11. Schinziophyton rautanenii Schinz 4.12. Sclerocarya birrea Sond. 4.13. Sesamum indicum L. 4.14. Sideroxylon inerme L. 4.15. Ximenia americana L. 5. Activities attributed to skin-care ethnobotanicals 5.1. Antioxidant activity 5.2. Anti-inflammatory activity 5.3. -

Eriocephalus Africanus: a Review of Its Medicinal Uses, Phytochemistry and Biological Activities
Alfred Maroyi /J. Pharm. Sci. & Res. Vol. 11(10), 2019, 3539-3546 Eriocephalus africanus: A review of its medicinal uses, phytochemistry and biological activities Alfred Maroyi Department of Biodiversity, University of Limpopo, Private Bag X1106, Sovenga 0727, South Africa. Abstract Eriocephalus africanus is a small shrub widely used as traditional medicine in South Africa. This study is aimed at providing a critical review of the botany, medicinal uses, phytochemistry and biological activities of E. africanus. Documented information on the botany, medicinal uses, phytochemistry and biological activities of E. africanus was collected from several online sources which included BMC, Scopus, SciFinder, Google Scholar, Science Direct, Elsevier, Pubmed and Web of Science. Additional information on the botany, medicinal uses, phytochemistry and biological activities of E. africanus was gathered from pre-electronic sources such as book chapters, books, journal articles and scientific publications obtained from the University library. This study showed that the leaves, oil and stems of E. africanus are mainly used as aromatherapy, colic, cosmetic, diaphoretic, diuretic, haemostatic, and herbal medicine for headache, swellings, dropsy, flatulence, heart problems, menstrual problems, footbath and dandruff, pain, dermal problems, fever, gynaecological problems, respiratory problems and stomach problems. Phytochemical compounds that have been identified from the aerial parts, leaves and stems of E. africanus include essential oils, flavonoids, sesquiterpenoid lactones, tannins and triterpene steroids. Pharmacological research revealed that the leaf, root, stem and twig extracts of E. africanus and essential oils isolated from the species have analgesic, antibacterial, antimycobacterial, antifungal, anti-inflammatory, antioxidant, antipyretic and phytotoxic activities. There is need for experimental animal studies, randomized clinical trials and target- organ toxicity studies involving E. -

The Effect of Isolated and Nanoencapsulated Flavonoids from Eriocephalus Africanus on Apoptotic Factors and Microrna Expression
The effect of isolated and nanoencapsulated flavonoids from Eriocephalus africanus on apoptotic factors and microRNA expression in cancer by Judie Magura Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy in Medical Sciences (Human Physiology) School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Westville, South Africa. Supervisor: Professor Irene Mackraj Co-supervisor: Dr Roshila Moodley June 2020 PREFACE The research work described in this thesis was conducted by the candidate at the University of KwaZulu-Natal, Westville, South Africa under the supervision of Professor Irene Mackraj and co-supervision of Dr Roshila Moodley. The contents of this work have not been submitted in any form for any degree to another University, and where use has been made of the work of others, it is duly acknowledged in the text, the results reported are from investigations by the candidate. _____________________ Signed: Judie Magura Date: 30 June 2020 _____________________ Signed: Prof Irene Mackraj (Supervisor) Date: 30 June 2020 _____________________ Signed: Dr Roshila Moodley (Co-supervisor) Date: 30 June 2020 i DECLARATION 1: PLAGIARISM I, Judie Magura, declare that: 1. The research reported in this dissertation, except where otherwise indicated or acknowledged, is my original work. 2. This dissertation has not been submitted in part or in full for any degree or examination at any other university. 3. This dissertation does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This dissertation does not contain other persons' writing, unless specifically acknowledged as being sourced from other researchers. -

The Potential of South African Plants in the Development of New Medicinal Products ⁎ B.-E
Available online at www.sciencedirect.com South African Journal of Botany 77 (2011) 812–829 www.elsevier.com/locate/sajb Review The potential of South African plants in the development of new medicinal products ⁎ B.-E. Van Wyk Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park 2006, South Africa Received 2 July 2011; received in revised form 26 August 2011; accepted 26 August 2011 Abstract Southern Africa is an important focal point of botanical and cultural diversity but only a few plant species have hitherto become fully commer- cialised as medicinal products. In recent years there has been an upsurge in research and development activity, resulting in several new products and new crops. In this review, more than 90 of the best-known and most promising indigenous South African plants are listed and subjectively evaluated in the context of their potential for commercialisation as medicinal products for a variety of applications. The history of product devel- opment relating to the following species is briefly discussed and the plants and some of their products are illustrated: Agathosma betulina (buchu), Aloe ferox (bitter aloe), Artemisia afra (African wormwood), Aspalathus linearis (rooibos tea), Bulbine frutescens (burn jelly plant); Cyclopia gen- istoides (honeybush tea), Harpagophytum procumbens (devil's claw), Hoodia gordonii (hoodia, ghaap), Hypoxis hemerocallidea (“African pota- to”), Lippia javanica (fever tea), Mesembryanthemum tortuosum (=Sceletium tortuosum)(kanna, kougoed), Pelargonium sidoides (“Umckaloabo”), Siphonochilus aethiopicus (African ginger), Sutherlandia frutescens (=Lessertia frutescens) (cancer bush), Warburgia salutaris (pepperbark tree) and Xysmalobium undulatum (“Uzara”). The main factors that are apparently responsible for failure or success will be highlight- ed, especially the importance of marketing strategy, proof of concept and barriers to market entry. -
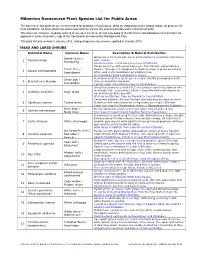
Plant List Are Recommended for Planting in Royal Ascot
1 Milnerton Racecourse Plant Species List for Public Areas The species on this plant list are recommended for planting in Royal Ascot. Most are indigenous to the fynbos region; all grow well in local conditions. All these plants are water-wise and can survive dry summer periods with a minimum of water. This list is not exclusive, anybody wishing to use species not on the list may apply to the Environmental Management Committee for approval in terms of section 2.6(g) of the Operational Environmental Management Plan. This plant list was revised in January 2012, changed species names were updated in January 2013. TREES AND LARGE SHRUBS Botanical Name Common Name Description & Natural Distribution Grows up to 15 m tall with sweet scented flowers in summer and silvery Sweet thorn / 1 Acacia karroo white thorns. Soetdoring Southern Africa – from low-lying areas to highveld. Fat growing tree with yellow bark up to 15m tall with yellow ball-like Fever tree / flowers. Athough not indigenous to Western Cape, it grows well and is 2 Acacia xanthophloea Koorsboom often used in city landscapes as a feature tree. From KwaZulu Natal norhtwards to Kenya. Silver oak / A shrub or small tree up to 10m in height. Usually developing a multi- 3 Brachylaena discolor stem or branching low down. Kusvaalbos Coastal region from Eastern Cape to Mozambique. Shrub that grows to a height of 5 m bearing cream to lilac flowers with an orange ‘eye’. In summer/ autumn. Sage-like dark green leaves on 4 Buddleja salviifolia Sage wood top and silvery white beneath. -

Fragrant Plants in the Garden PZA Article.Cdr
South African National Biodiversity Institute Gardening with South African Plants Fragrant plants in the garden By Alice Notten, Interpretation Ofcer, Kirstenbosch National Botanical Garden February 2017 Fragrance adds an extra dimension to the garden. When we pay attention to the scents and aromas wafting around us, a new world opens up. Scents can change a mood, induce feelings, trigger memories, make new ones, and entice one down the garden path. Clever use of scented and aromatic plants can turn any garden into an enchanting place. Fragrant foliage The core of a fragrant garden has to be plants with aromatic foliage. Most aromatic plants release their scent when touched, so plant them where they are most likely to be brushed against, such as beside paths, steps and gateways, in between paving stones, or in easy reach beside a bench, in raised beds or in window boxes. Some also scent the air around them, especially on warm, windless days. Next time you are shopping for plants, rub their leaves and have a sniff. Pick out those with scents that you like. There are many to choose from: Pelargoniums are rewarding garden plants with showy owers and beautiful foliage, and many have aromatic leaves, in an astonishing range of scents, including rose (Pelargonium capitatum, P. graveolens and P. radens), peppermint (P. tomentosum), lemon (P. citronellum and P. crispum), camphor (P. exstipulatum and P. betulinum), nutmeg (P. ‘Fragrans’) and balsam (P. glutinosum, P. panduriforme and P. quercifolium), and many more have their own unique fragrance. Add them to sunny beds and borders, and give them well-composted soil and moderate water. -
01.JPM TESI.Pdf
UNIVERSITAT DE BARCELONA FACULTAT DE FARMÀCIA DEPARTAMENT DE PRODUCTES NATURALS, BIOLOGIA VEGETAL I EDAFOLOGIA LABORATORI DE BOTÀNICA Sistemàtica i filogènia d’Artemisia i gèneres relacionats: una aproximació citogenètica i molecular amb especial èmfasi en el subgènere Dracunculus Jaume Pellicer Moscardó Barcelona, 2009 UNIVERSITAT DE BARCELONA FACULTAT DE FARMÀCIA DEPARTAMENT DE PRODUCTES NATURALS, BIOLOGIA VEGETAL I EDAFOLOGIA PROGRAMA DE DOCTORAT: MEDICAMENTS, ALIMENTACIÓ I SALUT BIENNI 2004-2006 Sistemàtica i filogènia d’Artemisia i gèneres relacionats: una aproximació citogenètica i molecular amb especial èmfasi en el subgènere Dracunculus Memòria presentada per Jaume Pellicer Moscardó per a optar al títol de Doctor per la Universitat de Barcelona Dr. Joan Vallès Xirau Dra. Teresa Garnatje Roca Jaume Pellicer Moscardó Barcelona, 2009 Aquest treball ha estat desenvolupat gràcies a una beca predoctoral FPI i tres borses de viatge per a estades breus concedides pel Ministeri de Ciència i Innovació. El finançament de les recerques ha anat a càrrec dels projectes dels Ministeris de Ciència i Tecnologia i Ciència i Innovació CGL2004-04563-C02- 02/BOS, CGL2007-64839-C02-01-01/BOS i CGL2007-64839-C02-01- 02/BOS, així com dels suports de la Generalitat de Catalunya a Grups de Recerca de Catalunya 2005/SGR/00344 i 2009/SGR/00439. Als meus pares i a la meva germana, naturalment Bé haguera estat trobar-nos a l’alçada impossible de la poesia de les nostres fonts, rierols i senderes, de la prosa plena i saborosa dels nostres vells màrgens, obagues i marjals, del ritme suau i primitiu dels nostres platjars i dunars, de la rudesa i cruor dels nostres cingles i runars, dels clímax dels nostres cims i abims.