Isotopic Composition of Nitrate and Particulate Organic Matter in a 2 Pristine Dam-Reservoir of Western India: Implications for 3 Biogeochemical Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Download
The Journal of Threatened Taxa (JoTT) is dedicated to building evidence for conservaton globally by publishing peer-reviewed artcles OPEN ACCESS online every month at a reasonably rapid rate at www.threatenedtaxa.org. All artcles published in JoTT are registered under Creatve Commons Atributon 4.0 Internatonal License unless otherwise mentoned. JoTT allows unrestricted use, reproducton, and distributon of artcles in any medium by providing adequate credit to the author(s) and the source of publicaton. Journal of Threatened Taxa Building evidence for conservaton globally www.threatenedtaxa.org ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) Note A new distribution record of the Western Ghats endemic damselfly Melanoneura bilineata Fraser, 1922 (Insecta: Odonata) from Maharashtra, India Yogesh Koli & Akshay Dalvi 26 August 2021 | Vol. 13 | No. 9 | Pages: 19380–19382 DOI: 10.11609/jot.7536.13.9.19380-19382 For Focus, Scope, Aims, and Policies, visit htps://threatenedtaxa.org/index.php/JoTT/aims_scope For Artcle Submission Guidelines, visit htps://threatenedtaxa.org/index.php/JoTT/about/submissions For Policies against Scientfc Misconduct, visit htps://threatenedtaxa.org/index.php/JoTT/policies_various For reprints, contact <[email protected]> The opinions expressed by the authors do not refect the views of the Journal of Threatened Taxa, Wildlife Informaton Liaison Development Society, Zoo Outreach Organizaton, or any of the partners. The journal, the publisher, the host, and the part- Publisher & Host ners are not responsible for -

A Geographical Analysis of Cashewnut Processing Industry in the Sindhudurg District, Maharashtra”
“A GEOGRAPHICAL ANALYSIS OF CASHEWNUT PROCESSING INDUSTRY IN THE SINDHUDURG DISTRICT, MAHARASHTRA” A Thesis Submitted to TILAK MAHARASHTRA VIDYAPEETH, PUNE For the Degree of Ph.D. Doctor of Philosophy (Vidyawachaspati ) in GEOGRAPHY Under the Faculty of Moral and Social Sciences by PATIL RAJARAM BALASO Lect. & Head Dept. of Geography Arts & Commerce College, Phondaghat Tal : Kankavli Dist : Sindhudurg UNDER THE GUIDANCE OF Dr. PRAVEEN G. SAPATARSHI Professor of Sustainability Management Indian Institute of Cost & Management Studies and Research, Pune APRIL 2010 DECLARATION I hereby declare that the thesis entitled “A GEOGRAPHICAL ANALYSIS OF CASHEW NUT PROCESSING INDUSTRY IN THE SINDHUDURG DISTRICT, MAHARASHTRA” completed and written by me has not previously formed the basis for the award of any Degree or other similar title of this or any other University or examining body. Place: Pune ( Shri. Rajaram B. Patil ) Date: 28-04-2010 Research student ii CERTIFICATE This is to certify that the thesis entitled “A GEOGRAPHICAL ANALYSIS OF CASHEWNUT PROCESSING INDUSTRY IN THE SINDHUDURG DISTRICT, MAHARASHTRA” which is being submitted herewith for the award of the Degree of Vidyawachaspati (Ph.D.) in Geography of Tilak Maharashtra Vidyapeeth, Pune is the result of the original research work completed by Shri. Rajaram Balaso Patil under my supervision and guidance. To the best of knowledge and belief the work incorporated in this thesis has not formed the basis for the award of any Degree or similar title of this or any other University or examining body. Place: Pune Dr. Praveen G. Saptarshi Date: 28-04-2010 Research Guide iii ACKNOWLEDGEMENTS While preparing this research work, numerous memories rush through my mind which is full of gratitude to those who encouraged and helped me at various stages. -

Self Study Report
SELF STUDY REPORT COVERING LETTER Laxmibai Sitaram Halbe College of Arts, Commerce and Science, Dodamarg 1 SELF STUDY REPORT S. N. PARTICULARS PAGE NO. 1 NAAC Steering Committee 04 2 Preface 05 3 Executive Summary 07 4 SWOC Analysis 12 5 Profile of the Institution 14 CRITERION- WISE ANALYTICAL REPORT 6 Criterion- I : Curricular Aspects 26 7 Criterion- II : Teaching-Learning and Evaluation 52 8 Criterion- III : Research, Consultancy and Extension 87 9 Criterion- IV : Infrastructure and Learning Resources 124 10 Criterion- V : Student Support and Progression 143 11 Criterion- VI : Governance, Leadership and Management 164 12 Criterion- VII : Innovations and Best Practices 192 INPUTS FROM THE DEPARTMENTS 13 Department of Marathi 207 14 Department of Hindi 213 15 Department of English 219 16 Department of Geography 225 17 Department of Economics 232 18 Department of Commerce 239 19 Declaration by the Head of the Institution 246 20 Certificate of Compliance 247 21 Annexure - I: Participation of Teachers in OP and RC 248 Laxmibai Sitaram Halbe College of Arts, Commerce and Science, Dodamarg 2 SELF STUDY REPORT 22 Annexure – II: Lay out Copy of the Library 250 23 Annexure – III: Affiliation Letter from the University 251 24 Annexure – IV: Letter of Change in the Name 252 25 Annexure – V: Certificate of Hilly Area 253 26 Annexure – VI: Master Plan of the Institution 254 27 Annexure – VII: Certificate of AISHE 256 28 Annexure – VIII: IEQA Application 257 29 Annexure –IX: Audit Reports 260 LOCATION OF THE COLLEGE Laxmibai Sitaram Halbe College of Arts, Commerce and Science, Dodamarg 3 SELF STUDY REPORT NAAC STEERING COMMITTEE Chairperson Dr. -

District Disaster Management Authority Sindhudurg
DISTRICT DISASTER MANAGEMENT PLAN SINDHUDURG UPDATED June 2020 DISTRICT DISASTER MANAGEMENT AUTHORITY SINDHUDURG Disaster Management Programme Govt.Of Maharashtra Executive Summary The District Disaster Management Plan is a key part of an emergency management. It will play a significant role to address the unexpected disasters that occur in the district effectively .The information available in DDMP is valuable in terms of its use during disaster. Based on the history of various disasters that occur in the district ,the plan has been so designed as an action plan rather than a resource book .Utmost attention has been paid to make it handy, precise rather than bulky one. This plan has been prepared which is based on the guidelines provided by the National Institute of Disaster Management (NIDM)While preparing this plan ,most of the issues ,relevant to crisis management ,have been carefully dealt with. During the time of disaster there will be a delay before outside help arrives. At first, self help is essential and depends on a prepared community which is alert and informed .Efforts have been made to collect and develop this plan to make it more applicable and effective to handle any type of disaster. The DDMP developed involves some significant issues like Incident Command System (ICS), India Disaster Resource Network (IDRN)website, the service of National Disaster Response Force (NDRF) in disaster management .In fact ,the response mechanism ,an important part of the plan is designed with the ICS, a best model of crisis management has been included in the response part for the first time. It has been the most significant tool to the response manager to deal with the crisis within the limited period and to make optimum use of the available resources. -

A Geographical Analysis of Major Tourist Attraction in Sindhudurg District, Maharashtra, India
Geoscience Research ISSN: 0976-9846 & E-ISSN: 0976-9854, Volume 4, Issue 1, 2013, pp.-120-123. Available online at http://www.bioinfopublication.org/jouarchive.php?opt=&jouid=BPJ0000215 A GEOGRAPHICAL ANALYSIS OF MAJOR TOURIST ATTRACTION IN SINDHUDURG DISTRICT, MAHARASHTRA, INDIA RATHOD B.L.1, AUTI S.K.2* AND WAGH R.V.2 1Kankawali College Kankawali- 416 602, MS, India. 2Art, Commerce and Science College, Sonai- 414 105, MS, India. *Corresponding Author: Email- [email protected] Received: October 12, 2013; Accepted: December 09, 2013 Abstract- Sindhudurg District has been declared as a 'Tourism District' on 30th April 1997. The natural resources, coastal lines, waterfalls, hot springs, temples, historical forts, caves, wild-life, hill ranges, scenery and amenable climate are very important resources of tourist attrac- tion. The various facilities available to the domestic and foreign tourists in Sindhudurg district. These include natural resources, transportation, infrastructure, hospitality resources and major tourist attractions. For the research work Sindhudurg District is selected. This district has at East Kolhapur district, at south Belgaum and Goa state at North Ratnagiri district and at west Arabian Sea. It is smallest district in Maharashtra state. It's area is 5207 sq.kms. Its geographical Location of Sindhudurg is 150 36' to 160 40' North latitudes as 730 19 to 740 18' East longitude. As per 2001 census it has 743 inhabited villages and 5 towns. The object of study region is, to highlight the attractive tourist destinations and religious places in the region. This study based on primary and secondary data. Tourist attractions in the district as is, natural beauty, waterfall, umala, caves, temples, beaches, ports, forts, mini garden, rock garden, tracking, rock climbing, boating, valley crossing, wild life, festival's fairs, arts, handicrafts, creeks, lakes etc. -

Brief Industrial Profile of Sindhudurg District MSME
GOVERNMENT OF INDIA MINISTRY OF MSME. Mumbai Brief Industrial Profile of Sindhudurg District Carried out by MSME-Development Institute, Ministry of Micro & Medium Enterprises, Govt of India Kurla Andheri Road, Sakinaka, Mumbai -72. Tel: 28576090/3091/7166 Fax: 28578092. Email: [email protected] Website: www.. msmedimumbai.gov.in 1 Contents S.No Topic Page No 1. General Characteristics of the District 3 1.1 Location & Geographical Area. 4 1.2 Topography 4 1.3 Availability of Minerals 5 1.4 Forest 5 1.5 Administrative Status 5 2 District at a glance 5-6 2.1 Existing Status of Industrial Area in the District 7 3 Industrial Scenario of Sidhudurg District 8 3.1 Industry at a Glance. 8 3.2 Year Wise trend of Units Registered 8-9 3.3 Details of Existing Micro & Small Enterprises & Artisan Units in The 9 District 3.4 Large Scale Industries/Public Sector undertakings 09 3.5 Major Exportable Item 09 3.6 Growth Trend 10 3.7 Vendorisation/Ancillarisation of the Industry 10 3.8 Medium Scale Enterprises. 10 3.9 Service Enterprises 11-12 3.9.2 Potential Area for Service Industries 11-15 3.10 Potential for New MSMEs 15 4. Existing Clusters of Micro & Small Enterprise. 15 4.1 Details of Major Clusters Identified 15 4.1.1 Manufacturing Sector 15 4.1.2. Service Sector 15 4.2 Details of Identified Cluster 15 4.2.1 Sindhudurg Cashew Cluster 15-16 5. General issues raised by industry association during the course of 15 meeting 6. Steps to set up MSMEs 16 2 Brief Industrial Profile of Sindhudurg District 1. -

Mammals Make Use of Cashew Plantations in a Mixed Forest‑Cashew Landscape
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Mammals make use of cashew plantations in a mixed forest‑cashew landscape Rege, Anushka; Punjabi, Girish Arjun; Jathanna, Devcharan; Kumar, Ajith 2020 Rege, A., Punjabi, G. A., Jathanna, D., & Kumar, A. (2020). Mammals make use of cashew plantations in a mixed forest‑cashew landscape. Frontiers in Environmental Science, 8, 556942‑. doi:10.3389/fenvs.2020.556942 https://hdl.handle.net/10356/145758 https://doi.org/10.3389/fenvs.2020.556942 © 2020 Rege, Punjabi, Jathanna and Kumar. This is an open‑access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. Downloaded on 29 Sep 2021 11:09:42 SGT fenvs-08-556942 November 21, 2020 Time: 15:20 # 1 ORIGINAL RESEARCH published: 26 November 2020 doi: 10.3389/fenvs.2020.556942 Mammals Make Use of Cashew Plantations in a Mixed Forest–Cashew Landscape Anushka Rege1,2,3*†, Girish Arjun Punjabi4†, Devcharan Jathanna5† and Ajith Kumar6,7 1 Post-graduate Programme in Wildlife Biology and Conservation, Wildlife Conservation Society India Program and National Centre for Biological Sciences, Bengaluru, India, 2 Asian School of the Environment, Nanyang Technological University, Singapore, Singapore, 3 Earth Observatory of Singapore, Nanyang Technological University, Singapore, Singapore, 4 Wildlife Conservation Trust, Mumbai, India, 5 Wildlife Conservation Society-India, Bengaluru, India, 6 National Centre for Biological Sciences, Bengaluru, India, 7 Centre for Wildlife Studies, Bengaluru, India Edited by: Heterogeneous landscapes harboring mosaics of natural habitat and agriculture are John A. -
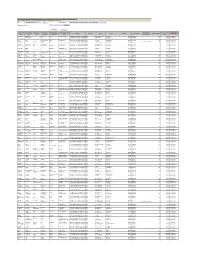
List of Shareholders Whose Shares Are Transferred to IEPF- 2009-10
Note: This sheet is applicable for uploading the particulars related to the shares transferred to Investor Education and Protection Fund. Make sure that the details are in accordance with the information already provided in e-form IEPF-4. CIN L24121MH1979PLC021360 Prefill Company Name DEEPAK FERTILISERS AND PETROCHEMICALS CORPORATION LTD Nominal value of shares 4734900.00 Validate Clear Actual Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Nominal value of Address Country State District Pin Code Folio Number Number of shares transfer to IEPF (DD- Name Name Name First Name Middle Name Name Account Number shares MON-YYYY) ARCHANA RAIZADA PREM SINGH RAIZADA STUDENT C/O MRS LATA RAIZADA D-28 74 BINDIA MADHYA PRADESH BHOPAL 462003 P175722 50 500.00 31-OCT-2017 D ANBALAGAN NA 39/F RAILWAY COLONY KODAMBAINDIA TAMIL NADU CHENNAI 600024 P188862 50 500.00 31-OCT-2017 MOHAMMED ALIM MOHAMMED SALIM SALES EXECUTIV C/O GODREJ 31 JANPATH BAPUJI NINDIA ORISSA BHUBANESHWAR 751009 P280406 75 750.00 31-OCT-2017 DIPTI SHAH PRANAYKUMAR SHAH HOUSE HOLD NARAYAN GULBAI TEKRA CHINUBHINDIA GUJARAT AHMEDABAD 380006 P124762 50 500.00 31-OCT-2017 GURMUKH SINGH SIDHU KISHAN SINGH 103 R MODEL TOWN LUDHIANA INDIA PUNJAB LUDHIANA 141002 IN301143-IN301143-10 75 750.00 31-OCT-2017 DOUGLAS C LAMOND ROBERT M LAMOND BUSINESS 19/2/A MANISH DEEP CO OP SOCIEINDIA MAHARASHTRA MUMBAI 400058 P223346 150 1500.00 31-OCT-2017 KISHORE NARBHERAM BAVISHI NARBHERAM N BAVISHI SERVICE 8-JANJIRA HOUSE RAJAWADI ROAINDIA -

Final Project Completion Report
CEPF SMALL GRANT FINAL PROJECT COMPLETION REPORT Organization Legal Name: - Ecological and Anthropogenic Correlates influencing Large Carnivore Occupancy and Project Title: Distribution in the Sahyadri – Konkan Corridor Date of Report: 2nd March 2014 Report Author and Contact Advait Edgaonkar, Indian Institute of Forest Information Management Bhopal CEPF Region: Western Ghats and Srilanka Biodiversity Hotspot Strategic Direction: Strategic Direction 2. Improve the conservation of globally threatened species through systematic conservation planning and action Grant Amount: $19,721 Project Dates: August 2009-July 2011 Implementation Partners for this Project (please explain the level of involvement for each partner): Center for Wildlife Studies, Bangalore:- provided logistics support, including support for research permission, library facilities and desk space for Principal Investigator Conservation Impacts Please explain/describe how your project has contributed to the implementation of the CEPF ecosystem profile. The distribution and occurrence of the tiger, dhole, sloth bear and leopard in the North Western Ghats and adjoining low elevation forests of the Konkan region in the state of Maharashtra, part of the Sahyadri - Konkan corridor (Critical Ecosystem Partnership Fund) was investigated and ecological and anthropogenic factors influencing this distribution and occurrence was quantified. As a result of the survey, six key areas of importance to the conservation of large carnivores were identified (See Annexure 1) Since this was a research project without an implementation component, the primary output was a recommendation for strengthening of conservation action in specific areas (given in Annexure 1). Locations (with geographical coordinates) have been provided where necessary action can be taken to allow potential movement of carnivores between protected areas and strengthen connectivity. -

BID DOCUMENT E-Tender for OFC Cable Laying Work in Sawantwadi and Vengurla Sdcas of SINDHUDURG SSA for Phase-VIII
BSNL/SNDG/T-92/ OFC CABLE LAYING WORK /SWDVGL/2017-18/03 Dated: 07/10/2017 1 | P a g e BID DOCUMENT e-Tender for OFC Cable Laying work in Sawantwadi and Vengurla SDCAs of SINDHUDURG SSA for Phase-VIII NIT No: BSNL/SNDG/T-92/OFC Cable Laying Work/SWDDMGVGL/2017-18/03 Dated: 07/10/2017 भारत संचार निगम लिलमटेड BHARAT SANCHAR NIGAM LIMITED (A Govt. of India Enterprise) OFFICE OF THE GENERAL MANAGER Sindhudurg SSA Sl Name of Work Estimate EMD Rs. Date & time of No Amount Rs. Bid opening OFC Cable Laying Work in Sawantwadi and Vengurla Rs. 02/11/2017 at 1 Rs.95,46,006 /- SDCAs of Sindhudurg SSA for 1,90,920/- 15:00 hrs Phase-VIII Note: Dodamarg Taluka area is included in Sawantwadi SDCAs. Tel. No. 02363-273333, Fax No. 02363-274780 E-mail: [email protected] website: www.eprocure.gov.in & www.maharashtra.bsnl.co.in Signature of bidder BSNL/SNDG/T-92/ OFC CABLE LAYING WORK /SWDVGL/2017-18/03 Dated: 07/10/2017 2 | P a g e BHARAT SANCHAR NIGAM LIMITED ( A Govt. of India Enterprise ) Office of The General Manager Sindhudurg Telephone Bhavan, Salaiwada, Sawantwadi Website: www.eprocure.gov.in & www.maharashtra.bsnl.co.in TABLE OF CONTENTS SECTION CONTENT PAGE NO QUALIFYING BID DOCUMENT I Notice Inviting Tender 3-6 II Bid Form 7-7 III Bidder's Profile 8-10 IV-A Instruction to Bidders 11-30 IV –B E-tendering Instructions to Bidders 31-34 V General (commercial) conditions of the contract 35-52 VI Special conditions of the contract 53-63 VII Scope of Work and jurisdiction of Contract 64-66 VIII Engineering instruction for OFC laying work -

~~~H ~ Ch1cfihl"1 FH~E-S
~~~H ~ Ch1cfihl"1 FH~e-s Rlfil~"~:flv:t WTfl1 : ~ <i'li~<Ri . <PR- 2 7, ~fkt~<J:><I"~<'I ~. ~ m. ~ ~-11o oo3 Indian Oil Corporation Limited Refineries Division : SCOPE Complex, Core-2 7, Institutional Area, Lodhi Road, New Delhi-110 003 Gram OILREFIN R'"h1 ~ 1f1 ~ >r'IWT Website : www.iocl.com Refineries Division Date: 05.07.2017 To, Member Secretary (lndustry-2), Ministry of Environment, Forest & Climate Change, Indira Paryavaran Bhawan, Jar Bagh Road New Delhi- 110003 Subject: Submission of replies to queries issued in MOM for Minutes of 24th Expert Appraisal Committee (lndustry-2) Meeting Held During 14th To 16th June 2017 - TOR for proposed West Coast Refinery and Petrochemical Project at Babulwadi, Rajapur (Ta luka), Ratnagiri (Dist), Maharashtra. Dear Sir, With reference to MOM for 24th EAC meeting, additional information sought by the committee is attached. This is for your submission and records Thanking you. Yours faithfully, eneral Manager Indian Oil Corporation Ltd. RHQ, New Delhi Enclosures: 1. Office memorandum and relevant pages of ESA notification 2. Location map 3. Letter from MIDC 4. Summary of site selection analysis 5. CD containing video coverage ~~ :\ilt-9 , afflT<rr<R~liflf , orr.w (~). ~-400 051('1U«r) Regd. Office G-9, Ali Yavar Jung Marg, Bandra (East) Mumbai-400 051 (India) ADDITIONAL INFORMATION SOUGHT BY EXPERT APPRAISAL COMMITTEE (EAC) AS PER MINUTES OF 24TH EAC (INDUSTRY-2) MEETING HELD DURING 14TH TO 16TH JUNE 2017 1. PP shall submit a letter from the concerned authority that the project does not fall in the ESA and the proposed activities are permitted in the area. -

MWDT Report Vol
MAHADAYI WATER DISPUTES TRIBUNAL THE REPORT-CUM-DECISION OF THE MAHADAYI WATER DISPUTES TRIBUNAL (Under Section 5(2) of The Inter-State River Water Disputes Act, 1956) IN THE MATTER OF REFERENCE NO. 1 OF 2011 RELATING TO WATER DISPUTES OF THE INTER-STATE RIVER MAHADAYI AND THE RIVER VALLEY THEREOF BETWEEN THE STATE OF GOA AND THE STATE OF KARNATAKA AND THE STATE OF MAHARASHTRA VOLUME - III (V O L U M E S I - XII) New Delhi 14th August 2018 REPORT OF THE MAHADAYI WATER DISPUTES TRIBUNAL I N D E X VOLUME – I Sl. No. D E S C R I P T I O N PAGE NOS 1. Introduction and Constitution of 1 - 6 Tribunal 2. Order dated 16.10.2012 passed by the 6 - 7 Tribunal in I.A. No.2 of 2012 filed by the State of Goa whereby directions were given to the three States to file their respective Statements of Claims alongwith Replies and Rejoinders 3. Chronological details of the Statement 7- 9 of cases/claims filed by the States of Goa, Maharashtra and Karnataka, along with Replies and Rejoinders. 4. Issues originally framed by the Tribunal 9 - 18 vide Order dated 21.8.2013. 5. Visit of the Tribunal to different sites 19 - 46 and the Report dated 12.02.2014 of the Assessors on the visits of the Tribunal. 6. Order dated 04.03.2014 passed by the 46 - 47 Tribunal in I.A. No. 19 of 2014 filed by the State of Goa for Amendment of Statement of claims and the consequential Pleadings of the Party States.