United States Patent Office Patented Jan
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation
Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation Roger Atkinson, Air Pollution Research Center, University of California, Riverside Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise 20% of the non- methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of these presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4- dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5- dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented. Live cast on the link below: http://www.fin.ucar.edu/it/mms/fl-live.htm A recording will be available on ACD’s website for viewing at a later date. -

United States Patent (19) (11) 3,855,105 Diveley (45) Dec
United States Patent (19) (11) 3,855,105 Diveley (45) Dec. 17, 1974 54 THOPHOSPHORYLATING ASATURATED 3,284,540 1 1/1966 D'Alelio.............................. 260/869 HYDROCARBON GROUP 75 Inventor: William R. Diveley, Oakwood Hills, Primary Examiner-Benjamin R. Padgett Del. Attorney, Agent, or Firm-George H. Hopkins 73 Assignee: Hercules incorporated, Wilmington, (57) ABSTRACT Del. Disclosed is a process for making certain organo thio 22) Filed: June 16, 1969 phosphates and dithiophosphates, a number of which 21 ) Appl. No.: 833,741 have utility as insecticides. The process comprises ef fecting by free radical catalysis at about 0-150°C. re Related U.S. Application Data action of an organic compound characterized by a sat (63) Continuation-in-part of Ser. No. 514,652, Dec. 17, urated carbon with at least one hydrogen replaceable 1965, abandoned. under free radical conditions, and halo-thiophosphate of the formula: 52 U.S. C. ..... 204/162 R, 204/158 R, 260/329 R, 260/329 P, 260/340.6, 260/347.2, 260/958, 7, OR . 1 / 260/963, 260/971 X-S-P (51) Int. Cl................................................ B01j 1/10 N 58 Field of Search ............ 204/162, 158; 260/329, OR 260/340.6, 347.2,958, 963,971 wherein X is a halo radical, Z is selected from the (56) References Cited group consisting of the oxo and thioxo radicals, and R UNITED STATES PATENTS and R' are organic radicals. 3,256,370 6/1966 Fitch et al........................... 260/972 24 Claims, No Drawings 3,855, 105 2 THIOPHOSPHORYLATING ASATURATED rated carbons, each of which has one or more hydrogen HYDROCARBON GROUP radicals replaceable under free radical conditions. -

Formation of Highly Oxygenated Organic Molecules from Aromatic Compounds
Formation of highly oxygenated organic molecules from aromatic compounds. Ugo Molteni1, Federico Bianchi1-2, Felix Klein1, Imad El Haddad1, Carla Frege1, Michel J. Rossi1, Josef Dommen1, Urs Baltensperger1,* 5 1Laboratory of Atmospheric Chemistry, Paul Scherrer Institute, CH-5232 Villigen, Switzerland 2Department of Physics, University of Helsinki, 00014 Helsinki, Finland Correspondence to: Urs Baltensperger ([email protected]) Abstract 10 Anthropogenic volatile organic compounds (AVOC) often dominate the urban atmosphere and consist to a large degree of aromatic hydrocarbons (ArHC), such as benzene, toluene, xylenes, and trimethylbenzenes, e.g. from handling and combustion of fuels. These compounds are important precursors for the formation of secondary organic aerosol. Despite their recognized importance as atmospheric reactants, the formation of highly oxygenated molecules (HOMs) in the gas phase leading to (extremely) low volatility compounds has not been studied in the past. Here we show that oxidation of 15 aromatics with OH leads to a subsequent autoxidation chain reaction forming HOMs with an O:C ratio of up to 1.09. This is exemplified for five single-ring ArHC (benzene, toluene, o-/m-/p-xylene, mesitylene (1,3,5-trimethylbenzene) and ethylbenzene), as well as two conjugated polycyclic ArHC (naphthalene and biphenyl). We present the identified compounds, differences in the observed oxidation patterns and discuss mechanistic pathways. We report the elemental composition of the HOMs and show the differences in the oxidation patterns of these ArHCs. A potential pathway for the 20 formation of these HOMs from aromatics is presented and discussed. We hypothesize that AVOC may contribute substantially to new particle formation events that have been detected in urban areas. -

Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers)
Development Support Document Final, September 4, 2015 Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers) Prepared by Joseph T. Haney, Jr., M.S. Angela Curry, M.S. Toxicology Division Office of the Executive Director TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Trimethylbenzenes Page i TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................................ I LIST OF TABLES ......................................................................................................................................................II ACRONYMS AND ABBREVIATIONS ................................................................................................................. III CHAPTER 1 SUMMARY TABLES .......................................................................................................................... 1 CHAPTER 2 MAJOR SOURCES AND USES ......................................................................................................... 4 CHAPTER 3 ACUTE EVALUATION ...................................................................................................................... 4 ACUTE 3.1 HEALTH-BASED ACUTE REV AND ESL ........................................................................................................ 4 3.1.1 Physical/Chemical Properties .................................................................................................................... -

Friedel-Crafts Alkylation of Aromatic Compounds with Phosphorus Estersla B
ALKYLATION OF AROMATIC COMPOUNDS WITH PHOSPHORUS ESTERS 1339 Analyse der Verbindungen: Die Einlagerungsverbin- Kalium-titandisulfid: Kalium 21,0%, Titan 33, 8%, dungen wurden vorsichtig mit HN03 aufgeschlossen. Schwefel 44,2%, Summe: 99,0%. Zusammen- Das im Fall der Wolframverbindungen dabei ausfal- setzung: Ko^TiSj,^ . lende W03 wurde alkalisch gelöst. Wolfram und Mo- Reaktionsprodukt von WS* mit Li-naphthalid (Uber- lybdän wurden als Oxinat, Titan als TiOa und Schwe- schuß) : Lithium 9,8%, Wolfram 66,3%, Schwefel fel als BaS04 bestimmt. Die Bestimmung der Alkali- 23,5%, Summe: 99,6%. Verhältnis 1 W : 2 Lili95S. metalle erfolgte flammenphotometrisch. Reaktionsprodukt WS2 mit Na-naphthalid (Überschuß) : Kalium-wolf ramdisulfid: Präparat I: Kalium 8,3%, Natrium 26,9%, Wolfram 53,1%, Schwefel 18,8%, Wolfram 66,9%, Schwefel 23,7%, Summe: 98,9%. Summe: 98,8%. Verhältnis: 1 W : 2 Na2,0S. Zusammensetzung: K0.59WS2)0 . Präparat II: Kalium 8,1%, Wolfram 67,1%, Schwe- fel 23,4%, Summe: 98,6%. Zusammensetzung: K0.57WS2,O . Kalium-molybdändisulfid: Kalium 10,86%, Molybdän Wir danken der Deutschen Forschungsgemeinschaft 53,6%, Sdiwefel 35,5%, Summe: 99,9%. Zusam- und dem Fonds der Chemie für die Unterstützung die- mensetzung: K0!49MoS1?98 . ser Arbeit. 1 Auszug aus der Dissertation E. BAYER. Tübingen 1970. 5 T. E. HOVEN-ESCH U. J. SMID, J. Amer. chem. Soc. 87, 669 2 W. RÜDORFF, Chimia [Zürich] 19,489 [1965], [1965]. 3 H. M. SICK, Dissertation Tübingen 1959. 6 W. BILTZ, P. EHRLICH U. M. MEISEL, Z. anorg. allg. Chem. 4 C. STEIN. J. POULENARD, L. BONNETAIN U. J. GOLE, C. R. 234,97 [1934], hebd. -

A Particular Focus on P-Cymene
materials Review Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene Anna Marchese 1, Carla Renata Arciola 2,3, Ramona Barbieri 1, Ana Sanches Silva 4,5, Seyed Fazel Nabavi 6, Arold Jorel Tsetegho Sokeng 7, Morteza Izadi 8, Nematollah Jonaidi Jafari 8, Ipek Suntar 9, Maria Daglia 7,* ID and Seyed Mohammad Nabavi 6,* 1 Sezione di Microbiologia DISC-IRCCS San Martino-IST University of Genoa, 16132 Genoa, Italy; [email protected] (A.M.); [email protected] (R.B.) 2 Research Unit on Implant Infections, Rizzoli Orthopaedic Institute, via di Barbiano 1/10, 40136 Bologna, Italy; [email protected] 3 Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Via San Giacomo 14, 40126 Bologna, Italy 4 National Institute for Agricultural and Veterinary Research (INIAV), I.P., Vairão, 4480 Vila do Conde, Portugal; [email protected] 5 Center for Study in Animal Science (CECA), ICETA, University of Oporto, 4051-401 Oporto, Portugal 6 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran 19395-5487, Iran; [email protected] 7 Department of Drug Sciences, Medicinal Chemistry and Pharmaceutical Technology Section, University of Pavia, 27100 Pavia, Italy; [email protected] 8 Health Research Center, Baqiyatallah University of Medical Sciences, Tehran 19395-5487, Iran; [email protected] (M.I.); [email protected] (N.J.J.) 9 Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, Etiler, Ankara 06330, Turkey; [email protected] * Correspondence: [email protected] (M.D.); [email protected] (S.M.N.); Tel./Fax: +98-21-88617712 (M.D.); +39-0382-987388 (S.M.N.) Received: 25 July 2017; Accepted: 11 August 2017; Published: 15 August 2017 Abstract: p-Cymene [1-methyl-4-(1-methylethyl)-benzene] is a monoterpene found in over 100 plant species used for medicine and food purposes. -

Sources of Non-Methane Hydrocarbons in Surface Air in Delhi, India” Gareth J
Electronic Supplementary Material (ESI) for Faraday Discussions. This journal is © The Royal Society of Chemistry 2020 Supplement to “Sources of non-methane hydrocarbons in surface air in Delhi, India” Gareth J. Stewart, a Beth S. Nelson, a Will S. Drysdale, a W. Joe F. Acton, b Adam R. Vaughan, a James R. Hopkins, a,c Rachel E. Dunmore, a C. Nicholas Hewitt, b Eiko Nemitz, d Neil Mullinger, d Ben Langford, d Shivani, e Ernesto R. Villegas, f Ranu Gadi, e Andrew R. Rickard, a,c James D. Lee a,c and Jacqueline F. Hamilton. a a Wolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, York, YO10 5DD, UK b Lancaster Environment Centre, Lancaster University, Lancaster LA1 4YQ, UK c National Centre for Atmospheric Science, University of York, York, YO10 5DD, UK d UK Centre for Ecology and Hydrology, Bush Estate, Penicuik, EH26 0QB, UK e Indira Gandhi Delhi Technical University for Women, Kashmiri Gate, New Delhi, Delhi 110006, India f Centre for Atmospheric Science, School of Earth and Environmental Sciences, University of Manchester, Manchester, M13 9PL, UK ESI1 – Air quality index in Delhi Plot created with air quality information data downloaded from the US embassy, New Delhi, for 2018 using US EPA method for calculation of air quality index (AQI). 1 Figure S1. AQI for New Delhi measured at the US embassy during 2018. ESI2 – Mean, minimum and maximum mixing ratios Calculated over sample periods where both DC-GC-FID and GCxGC-FID were measuring (29/05/18 20:00 to 05/06/18 11:00 and 11/10/2018 22:00 to 27/10/18 17:00). -

List of Dangerous Goods in Numerical Order See Volume II 3.2.2 Table
CHAPTER 3.2 LIST OF DANGEROUS GOODS 3.2.1 Table A: List of dangerous goods in numerical order See Volume II 3.2.2 Table B: List of dangerous goods in alphabetical order See Volume II 3.2.3 Table C: List of dangerous goods accepted for carriage in tank vessels in numerical order Explanations concerning Table C: As a rule, each row of Table C of this Chapter deals with the substance(s) covered by a specific UN number or identification number. However, when substances belonging to the same UN number or identification number have different chemical properties, physical properties and/or carriage conditions, several consecutive rows may be used for that UN number or identification number. Each column of Table C is dedicated to a specific subject as indicated in the explanatory notes below. The intersection of columns and rows (cell) contains information concerning the subject treated in that column, for the substance(s) of that row: – The first four cells identify the substance(s) belonging to that row; – The following cells give the applicable special provisions, either in the form of complete information or in coded form. The codes cross-refer to detailed information that is to be found in the numbers indicated in the explanatory notes below. An empty cell means either that there is no special provision and that only the general requirements apply, or that the carriage restriction indicated in the explanatory notes is in force. The applicable general requirements are not referred to in the corresponding cells. Explanatory notes for each column: Column (1) “UN number/identification number” Contains the UN number or identification number: – of the dangerous substance if the substance has been assigned its own specific UN number or identification number, or – of the generic or n.o.s. -

Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon
Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon Photooxidation under Low NOX Conditions L. Li1,2, P. Tang1,2, S. Nakao1,2,3 and D. R. Cocker III1,2 5 [1] University of California, Riverside, Department of Chemical and Environmental Engineering, Riverside, CA 92507, USA [2] College of Engineering-Center for Environmental Research and Technology (CE- CERT), Riverside, CA 92507, USA [3] Currently at Clarkson University, Department of Chemical and Biomolecular 10 Engineering, Potsdam, NY 13699, USA Correspondence to: D. Cocker III ([email protected]) Abstract The molecular structure of volatile organic compounds (VOC) determines their oxidation pathway, directly impacting secondary organic aerosol (SOA) formation. This study 15 comprehensively investigates the impact of molecular structure on SOA formation from the photooxidation of twelve different eight- to nine-carbon aromatic hydrocarbons under low NOx conditions. The effects of the alkyl substitute number, location, carbon chain length and branching structure on the photooxidation of aromatic hydrocarbons are demonstrated by analyzing SOA yield, chemical composition and physical properties. Aromatic hydrocarbons, 20 categorized into five groups, show a yield order of ortho (o-xylene and o-ethyltoluene)> one substitute (ethylbenzene, propylbenzene and isopropylbenzene) > meta (m-xylene and m- ethyltoluene)> three substitute (trimethylbenzenes) > para (p-xylene and p-ethyltoluene). SOA yields of aromatic hydrocarbon photooxidation do not monotonically decrease when increasing alkyl substitute number. The ortho position promotes SOA formation while the 25 para position suppresses aromatic oxidation and SOA formation. Observed SOA chemical composition and volatility confirm that higher yield is associated with further oxidation. SOA chemical composition also suggests that aromatic oxidation increases with increasing alkyl substitute chain length and branching structure. -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2. -
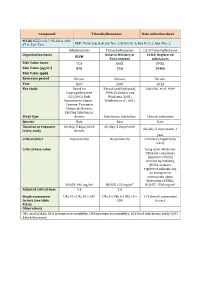
Compound Trimethylbenzenes Data Collection Sheet N°CAS
Compound Trimethylbenzenes Data collection sheet N°CAS 25551-13-7, 95-63-6, 108- 67-8, 526-73-8 CLP: Flam. Liq. 3, Acute Tox. 4, Skin Irrit. 2, Eye Irrit. 2, Asp. Tox. 1, Alkylbenzenes Trimethylbenzenes 1,2,4-Trimethylbenzene Organization Name Ontario Ministry of ECHA: Registered RIVM Environment substances Risk Value Name TCA AAQC DNEL Risk Value (µg/m3) 870 220 29400 Risk Value (ppb) Reference period Chronic Chronic Chronic Year 2007 2007 2013 Key Study Based on Korsak and Rydzynski, Clark DG, et al. 1989 Isopropylbenzene 1996; Gralewicz and EU (2001) Risk Wiaderna, 2001; Assessement Report – Wiaderna et al., 2002 Cumene. European Chemicals Bureau, Existing Substances. Study type chronic Subchronic inhalation Chronic inhalation Species Rats Rats Rats Duration of exposure 6h/day, 5 days/week 6h/day, 5 days/week 6h/day, 5 days/week, 1 in key study chronic year Critical effect Neurotoxicity Neurotoxicity Irritation (respiratory tract) Critical dose value Long term inhalation DNEL for consumers (systemic effects) derived by industry (ECHA-website: registered substances), no transparent information about derivation of DNEL NOAEL 490 mg/m3 NOAEL 123 mg/m³ NOAEC 1800 mg/m3 Adjusted critical dose 5.6 5.6 Single assessment UFH 10 x UFA 10 = 100 UFs 3 x UFH 3 x UFA 10 = 1.7 (Overall assessment factors (see table 100 factor) R.8.6) Other effects UFL used LOAEL; UFH intraspecies variability; UFA interspecies variability; UFS Used subchronic study; UFD data deficiencies Compound TRIMETHYLBENZENES Factsheet Parameter Note Comments Value / descriptor EU-LCI Value and Status EU-LCI value 1 Mass/volume [µg/m³] 450 EU-LCI status 2 Draft / Final Final Year when the EU-LCI value has been EU-LCI year of issue 3 2012 issued General Information 601-025-00-5 CLP-INDEX-Nr.