Provisional Peer-Reviewed Toxicity Values for Mixtures of Aliphatic And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation
Products of OH + Furan Reactions and Some Implications for Aromatic Hydrocarbon Atmospheric Degradation Roger Atkinson, Air Pollution Research Center, University of California, Riverside Unsaturated 1,4-dicarbonyls are important products of the atmospheric degradations of aromatic hydrocarbons such as benzene, toluene, xylenes and trimethylbenzenes, which comprise 20% of the non- methane volatile organic compounds present in urban air masses in the USA. However, in many cases the measured formation yields of the unsaturated 1,4-dicarbonyls are significantly lower than those of these presumed co-product 1,2-dicarbonyls. These discrepancies could be due to analytical problems and/or rapid photolysis of unsaturated 1,4-keto-aldehydes and unsaturated 1,4-dialdehydes, or to incorrect reaction mechanisms for the OH radical-initiated reactions of aromatic hydrocarbons. Since unsaturated 1,4- dicarbonyls are major products of OH + furans, with apparently simpler product distributions and mechanisms, we have investigated the reactions of OH radicals with furan, 2- and 3-methylfuran, and 2,3- and 2,5- dimethylfuran, in the presence of NO. Using direct air sampling atmospheric pressure ionization tandem mass spectrometry and gas chromatography, the unsaturated 1,4-dicarbonyls were observed and quantified. The measured unsaturated 1,4-dicarbonyl formation yields ranged from 8 ± 2% from OH + 2,3-dimethylfuran to 75 ± 5% from OH + furan. Other products were also formed. These data will be presented and discussed and, time permitting, a brief discussion of in situ nitro-aromatic and nitro-PAH formation from the atmospheric degradations of aromatic hydrocarbons and PAHs will also be presented. Live cast on the link below: http://www.fin.ucar.edu/it/mms/fl-live.htm A recording will be available on ACD’s website for viewing at a later date. -

Formation of Highly Oxygenated Organic Molecules from Aromatic Compounds
Formation of highly oxygenated organic molecules from aromatic compounds. Ugo Molteni1, Federico Bianchi1-2, Felix Klein1, Imad El Haddad1, Carla Frege1, Michel J. Rossi1, Josef Dommen1, Urs Baltensperger1,* 5 1Laboratory of Atmospheric Chemistry, Paul Scherrer Institute, CH-5232 Villigen, Switzerland 2Department of Physics, University of Helsinki, 00014 Helsinki, Finland Correspondence to: Urs Baltensperger ([email protected]) Abstract 10 Anthropogenic volatile organic compounds (AVOC) often dominate the urban atmosphere and consist to a large degree of aromatic hydrocarbons (ArHC), such as benzene, toluene, xylenes, and trimethylbenzenes, e.g. from handling and combustion of fuels. These compounds are important precursors for the formation of secondary organic aerosol. Despite their recognized importance as atmospheric reactants, the formation of highly oxygenated molecules (HOMs) in the gas phase leading to (extremely) low volatility compounds has not been studied in the past. Here we show that oxidation of 15 aromatics with OH leads to a subsequent autoxidation chain reaction forming HOMs with an O:C ratio of up to 1.09. This is exemplified for five single-ring ArHC (benzene, toluene, o-/m-/p-xylene, mesitylene (1,3,5-trimethylbenzene) and ethylbenzene), as well as two conjugated polycyclic ArHC (naphthalene and biphenyl). We present the identified compounds, differences in the observed oxidation patterns and discuss mechanistic pathways. We report the elemental composition of the HOMs and show the differences in the oxidation patterns of these ArHCs. A potential pathway for the 20 formation of these HOMs from aromatics is presented and discussed. We hypothesize that AVOC may contribute substantially to new particle formation events that have been detected in urban areas. -

Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers)
Development Support Document Final, September 4, 2015 Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers) Prepared by Joseph T. Haney, Jr., M.S. Angela Curry, M.S. Toxicology Division Office of the Executive Director TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Trimethylbenzenes Page i TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................................ I LIST OF TABLES ......................................................................................................................................................II ACRONYMS AND ABBREVIATIONS ................................................................................................................. III CHAPTER 1 SUMMARY TABLES .......................................................................................................................... 1 CHAPTER 2 MAJOR SOURCES AND USES ......................................................................................................... 4 CHAPTER 3 ACUTE EVALUATION ...................................................................................................................... 4 ACUTE 3.1 HEALTH-BASED ACUTE REV AND ESL ........................................................................................................ 4 3.1.1 Physical/Chemical Properties .................................................................................................................... -

Measurements of Higher Alkanes Using NO Chemical Ionization in PTR-Tof-MS
Atmos. Chem. Phys., 20, 14123–14138, 2020 https://doi.org/10.5194/acp-20-14123-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Measurements of higher alkanes using NOC chemical ionization in PTR-ToF-MS: important contributions of higher alkanes to secondary organic aerosols in China Chaomin Wang1,2, Bin Yuan1,2, Caihong Wu1,2, Sihang Wang1,2, Jipeng Qi1,2, Baolin Wang3, Zelong Wang1,2, Weiwei Hu4, Wei Chen4, Chenshuo Ye5, Wenjie Wang5, Yele Sun6, Chen Wang3, Shan Huang1,2, Wei Song4, Xinming Wang4, Suxia Yang1,2, Shenyang Zhang1,2, Wanyun Xu7, Nan Ma1,2, Zhanyi Zhang1,2, Bin Jiang1,2, Hang Su8, Yafang Cheng8, Xuemei Wang1,2, and Min Shao1,2 1Institute for Environmental and Climate Research, Jinan University, 511443 Guangzhou, China 2Guangdong-Hongkong-Macau Joint Laboratory of Collaborative Innovation for Environmental Quality, 511443 Guangzhou, China 3School of Environmental Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), 250353 Jinan, China 4State Key Laboratory of Organic Geochemistry and Guangdong Key Laboratory of Environmental Protection and Resources Utilization, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 510640 Guangzhou, China 5State Joint Key Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, 100871 Beijing, China 6State Key Laboratory of Atmospheric Boundary Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese -

Accuracy and Methodologic Challenges of Volatile Organic Compound–Based Exhaled Breath Tests for Cancer Diagnosis: a Systematic Review and Pooled Analysis
1 Supplementary Online Content Hanna GB, Boshier PR, Markar SR, Romano A. Accuracy and Methodologic Challenges of Volatile Organic Compound–Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Pooled Analysis. JAMA Oncol. Published online August 16, 2018. doi:10.1001/jamaoncol.2018.2815 Supplement. eTable 1. Search strategy for cancer systematic review eTable 2. Modification of QUADAS-2 assessment tools eTable 3. QUADAS-2 results eTable 6. STARD assessment of each study eTable 7. Summary of factors reported to influence levels of volatile organic compounds within exhaled breath eFigure 1. Risk of bias and applicability concerns using QUADAS-2 eFigure 2. PRISMA flowchart of literature search eTable 4. Details of studies on exhaled volatile organic compounds in cancer eTable 5. Cancer VOCs in exhaled breath and their chemical class. eFigure 3. Chemical classes of VOCs reported in different tumor sites. This supplementary material has been provided by the authors to give readers additional information about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 2 eTable 1. Search strategy for cancer systematic review # Search 1 (cancer or neoplasm* or malignancy).ab. 2 limit 1 to abstracts 3 limit 2 to cochrane library [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) Daily Update,Ovid MEDLINE(R) In-Process,Ovid MEDLINE(R) Publisher; records were retained] 4 limit 3 to english language 5 limit 4 to human 6 limit 5 to yr="2000 -Current" 7 limit 6 to humans 8 (cancer or neoplasm* or malignancy).ti. 9 limit 8 to abstracts 10 limit 9 to cochrane library [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) Daily Update,Ovid MEDLINE(R) In-Process,Ovid MEDLINE(R) Publisher; records were retained] 11 limit 10 to english language 12 limit 11 to human 13 limit 12 to yr="2000 -Current" 14 limit 13 to humans 15 7 or 14 16 (volatile organic compound* or VOC* or Breath or Exhaled).ab. -

APPENDIX a Speciated Compounds NMOC
APPENDIX A Speciated Compounds This appendix lists chemical species measured from the NMOC, PM2.5, and toxics monitoring programs. You can use this appendix to determine the corresponding AQS parameter code. For information on which sites monitor for speciated NMOC, PM2.5, or toxics data, see Table 1, "Air Quality Monitoring Data Availability". Compound Name AQS Parameter Code NMOC 1,2,3-Trimethylbenzene 45225 1,2,4-Trimethylbenzene 45208 1,3,5-Trimethylbenzene 45207 1,3-Butadiene 43218 1-Butene 43280 1-Pentene 43224 2,2,3-Trimethylbutane 43392 2,2,4-Trimethylpentane 43250 2,2-Dimethylbutane 43244 2,3,4-Trimethylpentane 43252 2,3-Dimethylbutane 43284 2,3-Dimethylpentane 43291 2,4-Dimethylpentane 43247 2,5-Dimethylhexane 43955 2-Methyl-1-Pentene 43246 2-Methyl-2-Butene 43228 2-Methylbutane 43221 2-Methylheptane 43960 2-Methylhexane 43263 2-Methylpentane 43285 3-Ethylhexane 43295 3-Methylbutene 43282 3-Methylheptane 43253 3-Methylhexane 43249 3-Methylpentane 43230 4-Mpentene/3-Mpentene 43391 Benzene 45201 Butane 43212 6 - 1 Compound Name AQS Parameter Code c-1,3-Dimethylcypentane 43393 c-2-Butene 43217 c-2-Hexene 43290 c-2-Pentene 43227 Cyclohexane 43248 Cyclopentane 43242 Cyclopentene 43283 Decane 43238 Ethane 43202 Ethene 43203 Ethylbenzene 45203 Ethyne 43206 Heptane 43232 Hexane 43231 Isobutane 43214 Isobutene 43270 Isoprene 43243 Iso-Propylbenzene 45210 m/p-Xylene 45109 m-Diethylbenzene 45218 Methylcyclohexane 43261 Methylcyclopentane 43262 m-Ethyltoluene 45212 Nonane 43235 n-Propylbenzene 45209 n-Undecane 43954 Octane 43233 o-Ethyltoluene -

Hydrocarbons, Bp 36°-216 °C 1500
HYDROCARBONS, BP 36°-216 °C 1500 FORMULA: Table 1 MW: Table 1 CAS: Table 1 RTECS: Table 1 METHOD: 1500, Issue 3 EVALUATION: PARTIAL Issue 1: 15 August 1990 Issue 3: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: cyclohexane n-heptane n-octane (Synonyms in Table 1) cyclohexene n-hexane n-pentane n-decane methylcyclohexane n-undecane n-dodecane n-nonane SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE [1] TECHNIQUE: GAS CHROMATOGRAPHY, FID [1] (coconut shell charcoal, 100 mg/50 mg) ANALYTE: Hydrocarbons listed above FLOW RATE: Table 3 DESORPTION: 1 mL CS2; stand 30 min VOL-MIN: Table 3 -MAX: Table 3 INJECTION VOLUME: 1 µL SHIPMENT: Routine TEMPERATURES SAMPLE -INJECTION: 250 °C STABILITY: 30 days @ 5 °C -DETECTOR: 300 °C -COLUMN: 35 °C (8 min) - 230 °C (1 min) BLANKS: 10% of samples ramp (7.5 °C /min) CARRIER GAS: Helium, 1 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Table 3 ID; 3.00-µm film 100% dimethyl polysiloxane BIAS: Table 3 CALIBRATION: Solutions of analytes in CS2 Ö OVERALL PRECISION ( rT): Table 3 RANGE: Table 4 ACCURACY: Table 3 ESTIMATED LOD: Table 4 þ PRECISION ( r): Table 4 APPLICABILITY: This method may be used for simultaneous measurements; however, interactions between analytes may reduce breakthrough volumes and alter analyte recovery. INTERFERENCES: At high humidity, the breakthrough volumes may be reduced. Other volatile organic solvents such as alcohols, ketones, ethers, and halogenated hydrocarbons are potential interferences. OTHER METHODS: This method is an update for NMAM 1500 issued on August 15, 1994 [2] which was based on methods from the 2nd edition of the NIOSH Manual of Analytical Methods: S28, cyclohexane [3]; S82, cyclohexene [3]; S89, heptane [3]; S90, hexane [3]; S94, methylcyclohexane [3]; S378, octane [4]; and S379, pentane [4]. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Flammable Liquefied Gas Mixture: 2-Methylpentane / 2,2-Dimethylbutane / 2, 3-Dimethylbutane / 3-Methylpentane / Benzene / Carbon Dioxide / Decane / Dodecane / Ethane / Ethyl Benzene / Heptane / Hexane / Isobutane / Isooctane / Isopentane / M- Xylene / Methane / N-Butane / N-Pentane / Neopentane / Nitrogen / Nonane / O- Xylene / Octane / P-Xylene / Pentadecane / Propane / Tetradecane / Toluene / Tridecane / Undecane Section 1. Identification GHS product identifier : Flammable Liquefied Gas Mixture: 2-Methylpentane / 2,2-Dimethylbutane / 2, 3-Dimethylbutane / 3-Methylpentane / Benzene / Carbon Dioxide / Decane / Dodecane / Ethane / Ethyl Benzene / Heptane / Hexane / Isobutane / Isooctane / Isopentane / M- Xylene / Methane / N-Butane / N-Pentane / Neopentane / Nitrogen / Nonane / O-Xylene / Octane / P-Xylene / Pentadecane / Propane / Tetradecane / Toluene / Tridecane / Undecane Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 018818 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Liquefied gas SKIN IRRITATION - Category 2 GERM CELL MUTAGENICITY - Category 1 CARCINOGENICITY - Category 1 TOXIC TO REPRODUCTION (Fertility) - Category 2 TOXIC TO REPRODUCTION (Unborn child) - Category 2 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) - Category 2 AQUATIC HAZARD (ACUTE) - Category 2 AQUATIC HAZARD (LONG-TERM) - Category 1 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. May form explosive mixtures with air. -

Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon
Impact of Molecular Structure on Secondary Organic Aerosol Formation from Aromatic Hydrocarbon Photooxidation under Low NOX Conditions L. Li1,2, P. Tang1,2, S. Nakao1,2,3 and D. R. Cocker III1,2 5 [1] University of California, Riverside, Department of Chemical and Environmental Engineering, Riverside, CA 92507, USA [2] College of Engineering-Center for Environmental Research and Technology (CE- CERT), Riverside, CA 92507, USA [3] Currently at Clarkson University, Department of Chemical and Biomolecular 10 Engineering, Potsdam, NY 13699, USA Correspondence to: D. Cocker III ([email protected]) Abstract The molecular structure of volatile organic compounds (VOC) determines their oxidation pathway, directly impacting secondary organic aerosol (SOA) formation. This study 15 comprehensively investigates the impact of molecular structure on SOA formation from the photooxidation of twelve different eight- to nine-carbon aromatic hydrocarbons under low NOx conditions. The effects of the alkyl substitute number, location, carbon chain length and branching structure on the photooxidation of aromatic hydrocarbons are demonstrated by analyzing SOA yield, chemical composition and physical properties. Aromatic hydrocarbons, 20 categorized into five groups, show a yield order of ortho (o-xylene and o-ethyltoluene)> one substitute (ethylbenzene, propylbenzene and isopropylbenzene) > meta (m-xylene and m- ethyltoluene)> three substitute (trimethylbenzenes) > para (p-xylene and p-ethyltoluene). SOA yields of aromatic hydrocarbon photooxidation do not monotonically decrease when increasing alkyl substitute number. The ortho position promotes SOA formation while the 25 para position suppresses aromatic oxidation and SOA formation. Observed SOA chemical composition and volatility confirm that higher yield is associated with further oxidation. SOA chemical composition also suggests that aromatic oxidation increases with increasing alkyl substitute chain length and branching structure. -

N-Alkane Category: Decane, Undecane, Dodecane (CAS Nos
June 17, 2004 n-Alkane Category: decane, undecane, dodecane (CAS Nos. 124-18-5, 1120-21-4, 112-40-3) Voluntary Children’s Chemical Evaluation Program (VCCEP) Tier 1 Pilot Submission Docket Number OPPTS – 00274D American Chemistry Council n-Alkane VCCEP Consortium Sponsors: Chevron Phillips Chemical Company LP Sasol North America Inc. Shell Chemical LP June 17, 2004 TABLE OF CONTENTS Glossary of Terms 4 1. Executive Summary 5 2. Basis for Inclusion in the VCCEP Program 2.1 Total Exposure Assessment Methodology Data 10 2.2 Air Monitoring Data 11 2.3 How Sponsors Were Identified for the n-Alkane VCCEP Effort 12 3. Previous and On-Going Health Assessments 3.1 OECD SIDS/ICCA HPV Imitative 14 3.2 Total Petroleum Hydrocarbon Criteria Working Group 15 3.3 Hydrocarbon Solvent Guidance Group Values (GGVs) for Setting Occupational Exposure Limits (OELs) for Hydrocarbon Solvents 15 4. Regulatory Overview 4.1 CPSC Child-Resistant Packaging for Hydrocarbons 16 4.2 Occupational Exposure Limits 16 4.3 VOC Regulations 17 5. Product Overview 5.1 Physical, Chemical, and Environmental Fate Properties 18 5.2 Production of n-Alkanes 20 5.3 Uses for n-Alkane Products 20 5.4 Petroleum Products Which Contain n-Alkanes 21 6. Exposure Assessment 6.1 Summary 25 6.2 Non-Occupational Exposure 28 6.2.1 Indoor Sources of Exposure 6.2.2 Outdoor Source of Exposure 6.2.3 Unique Children’s Exposure 6.3 Integrated 24 hour Exposure 36 6.4 Occupational Exposure 36 6.5 Potential for Dermal and Oral Exposure 40 6.6 Selection of Exposure Scenarios and Exposure Concentrations 43 7. -
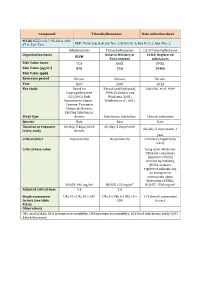
Compound Trimethylbenzenes Data Collection Sheet N°CAS
Compound Trimethylbenzenes Data collection sheet N°CAS 25551-13-7, 95-63-6, 108- 67-8, 526-73-8 CLP: Flam. Liq. 3, Acute Tox. 4, Skin Irrit. 2, Eye Irrit. 2, Asp. Tox. 1, Alkylbenzenes Trimethylbenzenes 1,2,4-Trimethylbenzene Organization Name Ontario Ministry of ECHA: Registered RIVM Environment substances Risk Value Name TCA AAQC DNEL Risk Value (µg/m3) 870 220 29400 Risk Value (ppb) Reference period Chronic Chronic Chronic Year 2007 2007 2013 Key Study Based on Korsak and Rydzynski, Clark DG, et al. 1989 Isopropylbenzene 1996; Gralewicz and EU (2001) Risk Wiaderna, 2001; Assessement Report – Wiaderna et al., 2002 Cumene. European Chemicals Bureau, Existing Substances. Study type chronic Subchronic inhalation Chronic inhalation Species Rats Rats Rats Duration of exposure 6h/day, 5 days/week 6h/day, 5 days/week 6h/day, 5 days/week, 1 in key study chronic year Critical effect Neurotoxicity Neurotoxicity Irritation (respiratory tract) Critical dose value Long term inhalation DNEL for consumers (systemic effects) derived by industry (ECHA-website: registered substances), no transparent information about derivation of DNEL NOAEL 490 mg/m3 NOAEL 123 mg/m³ NOAEC 1800 mg/m3 Adjusted critical dose 5.6 5.6 Single assessment UFH 10 x UFA 10 = 100 UFs 3 x UFH 3 x UFA 10 = 1.7 (Overall assessment factors (see table 100 factor) R.8.6) Other effects UFL used LOAEL; UFH intraspecies variability; UFA interspecies variability; UFS Used subchronic study; UFD data deficiencies Compound TRIMETHYLBENZENES Factsheet Parameter Note Comments Value / descriptor EU-LCI Value and Status EU-LCI value 1 Mass/volume [µg/m³] 450 EU-LCI status 2 Draft / Final Final Year when the EU-LCI value has been EU-LCI year of issue 3 2012 issued General Information 601-025-00-5 CLP-INDEX-Nr. -

HEATS of COMBUSTION and FORMATION of the PARAFFIN HYDROCARBONS at 25 C 1 by Edward J
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1642 Part of Journal of Research of the N.ational Bureau of Standards, Volume 34, March 1945 HEATS OF COMBUSTION AND FORMATION OF THE PARAFFIN HYDROCARBONS AT 25 C 1 By Edward j. Prosen and Frederick D. Rossini ABSTRACT Selected"best" values are given for the heats of combustion (in oxygen to form gaseous carbon dioxide and liquid water) and the heats of formation (from the elements solid carbon, graphite, and gaseous hydrogen) for methane and ethane in the gaseous state, and for all the paraffin hydrocarbons from propane through the octanes and the normal paraffins through eicosane, in both t he liquid (except for one octane which is solid) and gaseous states, all at 25° C. Equations are given for calculating values for all the normal paraffins above eicosane. CONTENTS Page L Introduction ____ ____________________ ___ ___ __________ ____ _______ _ 263 II. Unit of energy, molecular weights, etc ___ ______ ________ ___________ _ 264 IlL Experimental data considered __________ __ _________________ _____ ___ 264 IV. Selected values_ __ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __ _ _ __ __ _ _ __ _ _ _ _ _ _ _ _ _ _ _ _ 266 V. References ______ _____ ________________________ ______ __ __ ____ ___ __ 268 1. INTRODUCTION In 1940, values were presented by this Bureau for the heats of for mat.ion of the paraffin hydrocarbons from methane through the pen tanes, in the gaseous state [1).2 Since then, a considerable amount of new data has become available, which makes possible the extension of these values to all the isomers of the hexanes, heptanes, and octanes, and to the higher normal paraffins, each in both the liquid and gaseous states.