203629Orig1s000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiovascular Monitoring with Acetylcholinesterase Inhibitors: a Clinical Protocol† Jeremy P
Advances in Psychiatric Treatment (2007), vol. 13, 178–184 doi: 10.1192/apt.bp.106.002725 Cardiovascular monitoring with acetylcholinesterase inhibitors: a clinical protocol† Jeremy P. Rowland, John Rigby, Adam C. Harper & Rosalind Rowland Abstract There has been significant anxiety among prescribers regarding the potential for cardiac adverse effects associated with acetylcholinesterase (AChE) inhibitors in Alzheimer’s disease. There is no consensus on how to manage this cardiovascular risk, and memory clinics vary widely in their practice. Review of published evidence reveals that the incidence of cardiovascular side-effects is low, and that serious adverse events are rare. Intensive cardiovascular screening such as pre-treatment electrocardiograms or 24 h cardiac monitoring is not justified. Furthermore, there are no high-risk groups to target. This article suggests pragmatic guidelines for managing cardiovascular risk in patients receiving AChE inhibitors. The guidelines are intended to be easy to incorporate into routine clinical practice in a memory clinic. A few years ago it was estimated that almost 18 thus followed some uncertainty as to how treatment million people worldwide had dementia (Alzheimer’s should be properly managed. Society, 2004), with Alzheimer’s disease accounting Since the manufacturers’ cautions remain (see the for over half of cases (Fratiglioni, 2000). The second- relevant entries in http://emc.medicines.org.uk) and generation acetylcholinesterase (AChE) inhibitors guidance has been unforthcoming, services now vary donepezil, rivastigmine and galantamine were widely in their practice: some, for example, require introduced into clinical practice from 1997 for the electrocardiograms (ECGs) before use and during symptomatic treatment of Alzheimer’s disease, and treatment, whereas others do not. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

On Tardive Dyskinesia'
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.8.941 on 1 August 1974. Downloaded from Journial of Neurology, Neurosurgery, alid Psychiatry, 1974, 27, 941-947 Effect of cholinergic and anticholinergic agents on tardive dyskinesia' H. L. KLAWANS2 AND R. RUBOVITS Fr-om the Divisioni of Neurology, Michael Reese Medical Center, Chicago, Illinois anid the Departmentt ofPsychiatry, University of Maryland, Baltimore, Maryland, U.S.A. SYNOPSIS Tardive dyskinesia, like several other choreiform disorders, is felt to be primarily related to dopaminergic activity within the striatum. Physostigmine has been demonstrated to improve the abnormal movements in patients with tardive dyskinesia while scopolamine tends to aggravate abnormal movements and in some cases elicits abnormal movement not previously observed. This evidence supports the hypothesis that anticholinergic therapy in patients prone to develop tardive dyskinesia may increase the incidence of this disorder the threshold for the by lowering appearance guest. Protected by copyright. of these movements. Tardive dyskinesia is a well-recognized side- been fully elucidated. However, there is evidence effect of long-term neuroleptic therapy (Crane, which suggests that dopamine acting at striatal 1968). The most prominent manifestation dopaminergic receptor sites may be closely is lingual-facial-buccal dyskinesia. Limb and related to the initiation of these choreiform trunkal chorea may accompany the facial move- movements in several clinical settings. Drugs ments (Paulson, 1968). The syndrome is most which alter the availability of dopamine at often seen in patients ranging in age from 50 to dopaminergic receptor sites alter choreiform 70 years who are most often diagnosed as symptomatology. Huntington's chorea is re- suffering chronic deteriorating schizophrenia. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

ENLON-PLUS (Edrophonium Chloride, USP and Atropine Sulfate, USP) Injection
NDA 19-677/S-005 NDA 19-678/S-005 Page 3 ENLON-PLUS (edrophonium chloride, USP and atropine sulfate, USP) Injection Rx only DESCRIPTION ENLON-PLUS (edrophonium chloride, USP and atropine sulfate, USP) Injection, for intravenous use, is a sterile, nonpyrogenic, nondepolarizing neuromuscular relaxant antagonist. ENLON-PLUS is a combination drug containing a rapid acting acetylcholinesterase inhibitor, edrophonium chloride, and an anticholinergic, atropine sulfate. Chemically, edrophonium chloride is ethyl (m-hydroxyphenyl) dimethylammonium chloride; its structural formula is: Molecular Formula: C10H16ClNO Molecular Weight: 201.70 Chemically, atropine sulfate is: endo-(±)-alpha-(hydroxymethyl)-8-methyl-8-azabicyclo [3.2.1]oct-3-yl benzeneacetate sulfate (2:1) monohydrate. Its structural formula is: Molecular Formula: (C17H23NO3)2·H2SO4·H2O NDA 19-677/S-005 NDA 19-678/S-005 Page 4 Molecular Weight: 694.84 ENLON-PLUS contains in each mL of sterile solution: 5 mL Ampuls: 10 mg edrophonium chloride and 0.14 mg atropine sulfate compounded with 2.0 mg sodium sulfite as a preservative and buffered with sodium citrate and citric acid. The pH range is 4.0- 5.0. 15 mL Multidose Vials: 10 mg edrophonium chloride and 0.14 mg atropine sulfate compounded with 2.0 mg sodium sulfite and 4.5 mg phenol as a preservative and buffered with sodium citrate and citric acid. The pH range is 4.0-5.0. CLINICAL PHARMACOLOGY Pharmacodynamics ENLON-PLUS (edrophonium chloride, USP and atropine sulfate, USP) Injection is a combination of an anticholinesterase agent, which antagonizes the action of nondepolarizing neuromuscular blocking drugs, and a parasympatholytic (anticholinergic) drug, which prevents the muscarinic effects caused by inhibition of acetylcholine breakdown by the anticholinesterase. -

Integrated Approach for Identifying the Molecular, Cellular, and Host Responses to Chemical Insults
Integrated Approach for Identifying the Molecular, Cellular, and Host Responses to Chemical Insults Audrey E. Fischer, Emily P. English, Julia B. Patrone, Kathlyn Santos, Jody B. G. Proescher, Rachel S. Quizon, Kelly A. Van Houten, Robert S. Pilato, Eric J. Van Gieson, and Lucy M. Carruth e performed a pilot study to characterize the molecular, cellular, and whole-organism response to nonlethal chemical agent exposure in the central nervous system. Multiple methodologies were applied to measure in vitro enzyme inhibition, neuronal cell pathway signaling, and in vivo zebrafish neural development in response to challenge with two different classes of chemical compounds. While all compounds tested exhibited expected enzyme inhibitory activity against acetylcholinesterase (AChE), a well-characterized target of chemical agents, distinct differences between chemical exposures were detected in cellular toxicity and pathway target responses and were tested in a zebrafish model. Some of these differences have not been detected using conventional chemical toxicity screening methods. Taken together, the data demonstrate the potential value of an integrated, multimethodological approach for improved target and pathway identification for subsequent diagnostic and therapeutic biomarker development. INTRODUCTION To build capability and leverage new and growing cell models to complete living organisms. Regardless of biology and chemistry expertise at APL, a collabora- the model selected, challenges exist in sample collection, tive, cross-departmental effort was established through a dose determination, and biases inherent in each assay/ series of related independent research and development technology. Therefore, multiple experimental methodol- (IR&D) projects. The focus of this effort was on mitiga- ogies brought to bear on a particular biological question tion of chemical and biological threat agents. -

Carey Nat Pope
CAREY NAT POPE College of Veterinary Medicine Oklahoma State University 264 McElroy Hall Stillwater, OK 74078 [email protected] (405)744-6257 (fax)744-4345 EDUCATION 1981-1985 University of Texas Graduate School of Biomedical Sciences, Houston, TX. Degree: Ph.D. (Pharmacology/Toxicology). 1977-1979 Stephen F. Austin State University, Nacogdoches, TX. Degree: M.S. (Biology). 1974-1976 Stephen F. Austin State University, Nacogdoches, TX. Degree: B.S. (Biology). 1971-1973 University of Houston, Houston, TX. EXPERIENCE 12/2015-present Adjunct Professor, Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, OK 10/2014-present Adjunct Professor, Department of Integrative Biology, Oklahoma State University, Stillwater, OK 3/2013-present Director, Graduate Certificate Program in Interdisciplinary Toxicology, Oklahoma State University, Stillwater, OK. 6/2012-present Director, Interdisciplinary Toxicology Program, Oklahoma State University, Stillwater, OK. 7/1/2006-1/31/2012 Head, Department of Physiological Sciences, College of Veterinary Medicine, Oklahoma State University, Stillwater, OK. 8/1/2005-6/30/06 Interim Head, Department of Physiological Sciences, College of Veterinary Medicine, Oklahoma State University, Stillwater, OK. 1/2000-present Professor and Sitlington Endowed Chair in Toxicology, Department of Physiological Sciences, College of Veterinary Medicine, Oklahoma State University, Stillwater, OK. 8/95-12/99 Director, Division of Toxicology, College of Pharmacy and Health Sciences, University of Louisiana at Monroe, Monroe, LA. Division included five full-time tenured or tenure-track members and one instructor and was responsible for implementing B.S., M.S. and Ph.D. degree programs in Toxicology. 12/93-12/99 Director, B.S. Toxicology Program, College of Pharmacy and Health Sciences, University of Louisiana at Monroe, Monroe, LA. -
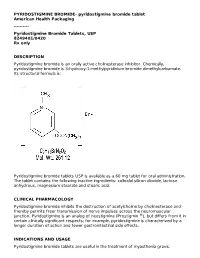
Pyridostigmine Bromide Tablets, USP 8249401/0420 Rx Only
PYRIDOSTIGMINE BROMIDE- pyridostigmine bromide tablet American Health Packaging ---------- Pyridostigmine Bromide Tablets, USP 8249401/0420 Rx only DESCRIPTION Pyridostigmine bromide is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is: Pyridostigmine bromide tablets USP is available as a 60 mg tablet for oral administration. The tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose anhydrous, magnesium stearate and stearic acid. CLINICAL PHARMACOLOGY Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin ®), but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects. INDICATIONS AND USAGE Pyridostigmine bromide tablets are useful in the treatment of myasthenia gravis. CONTRAINDICATIONS Pyridostigmine bromide is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below. WARNINGS Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. -

Mytelase (Ambenonium Chloride) Tablets Label
NDA 010155/S-022 NDA 010155/ S-023 FDA Approved Labeling Text dated 11/10/2011 Page 1 MYTELASE® AMBENONIUM CHLORIDE DESCRIPTION MYTELASE, brand of ambenonium chloride, is [Oxalylbis (iminoethylene)] bis[(o chlorobenzyl) diethylammonium] dichloride, a white crystalline powder, soluble in water to 20 percent (w/v). Inactive Ingredients: Acacia, Dibasic Calcium Phosphate, Gelatin, Lactose, Magnesium Stearate, Starch, Sucrose. CLINICAL PHARMACOLOGY The compound is a cholinesterase inhibitor with all the pharmacologic actions of acetylcholine, both the muscarinic and nicotinic types. Cholinesterase inactivates acetylcholine. Like neostigmine, MYTELASE suppresses cholinesterase but has the advantage of longer duration of action and fewer side effects on the gastrointestinal tract. The longer duration of action also results in more even strength, better endurance, and greater residual effect during the night and on awakening than is produced by shorter-acting anticholinesterase compounds. INDICATION AND USAGE This drug is indicated for the treatment of myasthenia gravis. CONTRAINDICATIONS Routine administration of atropine with MYTELASE is contraindicated since belladonna derivatives may suppress the parasympathomimetic (muscarinic) symptoms of excessive gastrointestinal stimulation, leaving only the more serious symptoms of fasciculation and paralysis of voluntary muscles as signs of overdosage. MYTELASE should not be administered to patients receiving mecamylamine, or any other ganglionic blocking agents. MYTELASE should also not be administered to patients with a known hypersensitivity to ambenonium chloride or any other ingredients of MYTELASE. WARNINGS Because this drug has a more prolonged action than other antimyasthenic drugs, simultaneous administration with other cholinergics is contraindicated except under strict medical supervision. The overlap in duration of action of several drugs complicates dosage schedules. -

Cholinergic Regulation of Neurite Outgrowth from Isolated Chick Sympathetic Neurons in Culture
The Journal of Neuroscience, January 1995, 15(i): 144-151 Cholinergic Regulation of Neurite Outgrowth from Isolated Chick Sympathetic Neurons in Culture David H. Small,’ Gullveig Reed,’ Bryony Whitefield,’ and Victor Nurcombe* Departments of ‘Pathology and 2Anatomy and Cell Biology, The University of Melbourne, and the Mental Health Research Institute of Victoria, Parkville, Victoria 3052, Australia Neurotransmitters have been reported to regulate neurite mate, serotonin, and dopamine have all beenshown to influence outgrowth in several vertebrate and nonvertebrate species. neurite outgrowth in culture (Mattson, 1988; Lipton and Kater, In this study, cultures of isolated embryonic day 12 (E12) 1989). There is also evidence that ACh could have nonclassical chick sympathetic neurons were grown in the presence of actions in the nervous system (Lankford et al., 1988; Lipton et cholinergic receptor agonists or antagonists. Both ACh and al., 1988; Mattson, 1988). The biosynthetic and degradative the nonhydrolyzable cholinergic agonist carbamylcholine enzymesofcholinergic pathways ChAT and AChE are expressed (CCh) inhibited neurite outgrowth. ACh (0.1-l .O mM) de- in the developing brain well before the major period of syn- creased the percentage of neurons bearing neurites, but had aptogenesis(Filogamo and Marchisio, 1971; Silver, 1974), sug- no significant effect on cell survival. The effect of ACh was gesting that they may be involved in functions unrelated to increased in the presence of the cholinesterase inhibitors neurotransmission. ACh has been shown to suppressneurite BW284C51 (1 MM), Tacrine (20 PM), and edrophonium (200 outgrowth from chick (Lankford et al., 1988) and rat (Lipton et PM). Neurite outgrowth was strongly inhibited by the mus- al., 1988) retinal cells, from hippocampal pyramidal neurons carinic receptor agonist oxotremorine (5-100 PM) and weakly (Mattson, 1988) and to prevent the inhibition of processout- inhibited by nicotine (50 nM to 10 PM). -

UNIVERSITY of CALIFORNIA SAN DIEGO Establishment and Validation of the Freshwater Planarian, Dugesia Japonica, As an Alternative
UNIVERSITY OF CALIFORNIA SAN DIEGO Establishment and Validation of the Freshwater Planarian, Dugesia japonica, as an Alternative Animal Model for Developmental Neurotoxicology using Organophosphorus Pesticides A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Biology by Danielle Hagstrom Committee in charge: Professor Eva-Maria Schoetz Collins, Chair Professor James Posakony, Co-Chair Professor Palmer Taylor Professor Robert Tukey Professor Jing Wang Professor Deborah Yelon 2018 Copyright Danielle Hagstrom, 2018 All rights reserved. The Dissertation of Danielle Hagstrom is approved, and it is acceptable in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California San Diego 2018 iii TABLE OF CONTENTS Signature Page………………………………………………………………………………... iii Table of Contents……………………………………………………………………………... iv List of Figures……………………………………………………………………………........ v List of Tables………………………………………………………………………………..... vii Acknowledgements………………………………………………………………………....... viii Vita…………………………………………………………………………………………… xii Abstract of the Dissertation…………………………………………………………………… xiii Chapter 1: Planarian brain regeneration as a model system for developmental neurotoxicology………….……………………………………………………………………. 1 Chapter 2: Freshwater planarians as an alternative animal model for neurotoxicology………. 38 Chapter 3: Multi-behavioral endpoint testing of an 87-chemical compound library in freshwater planarians………………………………………………………………………………………. 83 Chapter 4: Comparative -

PH 1.14 Parasympathomimetics Cholinergic Drugs Cholinergic and Adrenergic System
. At the end of this session, the student should be able to: • Describe cholinergic transmission • Enumerate types of cholinergic receptors, clinically relevant sites where present & response on stimulation PH 1.14 • Enumerate choline esters & alkaloids & mention clinical uses of each Parasympathomimetics with basis • Enumerate anticholinesterases, describe their important uses with Cholinergic drugs preferred agent & basis of use • Describe clinically relevant differences between Physostigmine & Neostigmine • Explain why Physostigmine is preferred for t/t of glaucoma & Belladona poisoning • Explain why Edrophonium is used for diagnostic purpose but not 1 preferred for therapeutic purpose 2 Parasympathetic Nervous System (Craniosacral Outflow) • Explain why anticholinesterases are not used for reversing the action SA & AV Node Bronchi/Bronchial of succinylcholine Circular Muscle of Iris Glands Ciliary Muscle • Explain t/t of early mushroom poisoning & acute organophosphate Stomach poisoning • Write a brief note on: Small Intestine Lacrimal Gland a) Edrophonium Bile Ducts b) Pralidoxime Gallbladder c) Cholinergic crisis Submaxillary & Kidney Sublingual d) Myasthenic crisis Glands Large Intestine Bladder Parotid Gland Genitalia 3 4 Sympathetic Nervous System (Thoracolumbar Outflow) Radial Muscle of Iris Ciliary Muscle Cholinergic and Adrenergic System Sublingual,Submaxillary & Parotid Gland • Accordingly: Pilomotor Muscles SA & AV Nodes Sweat Glands His-Purkinje System – Cholinergic Drugs, i.e. they act by releasing Myocardium acetylcholine Bronchi/Bronchial Glands • But also utilize nitric oxide (NO) or peptides for transmission Stomach Blood Vessels – Noradrenergic (commonly called "adrenergic") Drugs - Kidneys act by releasing norepinephrine (NA) Paravertebral Ganglia Intestines Bladder/ Genitalia Prevertebral Ganglia 5 6 1 . Sites of Cholinergic Transmission Schematic diagram comparing some anatomic 1. All preganglionic sites (Both Parasympathetic and sympathetic) and neurotransmitter features of autonomic and 2.