A Morphological and Molecular Study of Diversity and Connectivity Among Anchialine Shrimp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Extreme Phenotypic Variation Among Cryptic Caridean Shrimp from San Salvador Island, Bahamas
EXTREME PHENOTYPIC VARIATION AMONG CRYPTIC CARIDEAN SHRIMP FROM SAN SALVADOR ISLAND, BAHAMAS. Robert E. Ditter1, Anna M. Goebel2 and Robert B. Erdman2 1Department of Marine and Ecological Sciences 2Department of Biological Sciences Florida Gulf Coast University Ft. Myers, FL 33965 ABSTRACT the dissolution of the underlying carbonate plat- form (Edwards, 1996; Mylroie and Carew, 2003). Extensive phenotypic variation is docu- No study has successfully traced the lakes’ connec- mented in specimens of cryptic cave shrimp col- tion to the surrounding ocean on San Sal. Our lected on San Salvador Island, Bahamas in June of original objective was to compare species compo- 2012. Sixty two of the 70 individuals exhibited sition of cryptic caridean shrimp inhabiting the such immense variation, including characters not conduit mouths of anchialine pools and use this previously described to Barbouria cubensis, Bar- information to infer connections between lakes and bouria yanezi, Parhippolyte sterreri, or any mem- the surrounding ocean. ber of the family Barbouriidae, that identification using a traditional morphological approach proved impossible. Examples of such characters include terminal eyestalk spines, gross spination of the tel- son and the presence of sensory dorsal organs on the carapace. The source and extent of morpholog- ical variation in these shrimp remains unclear, but may be due to environmental or genetic causes, or a combination of both. We note that rampant vari- ation is observed in taxonomically important char- acters that were previously considered highly con- served and therefore were used to discriminate among species of shrimp. If these characters are highly variable then the taxonomic identity of shrimp in this group may need to be reconsidered. -

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

Anchialine Cave Biology in the Era of Speleogenomics Jorge L
International Journal of Speleology 45 (2) 149-170 Tampa, FL (USA) May 2016 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Life in the Underworld: Anchialine cave biology in the era of speleogenomics Jorge L. Pérez-Moreno1*, Thomas M. Iliffe2, and Heather D. Bracken-Grissom1 1Department of Biological Sciences, Florida International University, Biscayne Bay Campus, North Miami FL 33181, USA 2Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA Abstract: Anchialine caves contain haline bodies of water with underground connections to the ocean and limited exposure to open air. Despite being found on islands and peninsular coastlines around the world, the isolation of anchialine systems has facilitated the evolution of high levels of endemism among their inhabitants. The unique characteristics of anchialine caves and of their predominantly crustacean biodiversity nominate them as particularly interesting study subjects for evolutionary biology. However, there is presently a distinct scarcity of modern molecular methods being employed in the study of anchialine cave ecosystems. The use of current and emerging molecular techniques, e.g., next-generation sequencing (NGS), bestows an exceptional opportunity to answer a variety of long-standing questions pertaining to the realms of speciation, biogeography, population genetics, and evolution, as well as the emergence of extraordinary morphological and physiological adaptations to these unique environments. The integration of NGS methodologies with traditional taxonomic and ecological methods will help elucidate the unique characteristics and evolutionary history of anchialine cave fauna, and thus the significance of their conservation in face of current and future anthropogenic threats. -
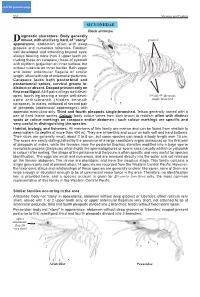
W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Lysmata Amboinensis (De Man, 1888)
Lysmata amboinensis (de Man, 1888) B. Santhosh, M. K. Anil and Biji Xavier IDENTIFICATION Order : Decapoda Family : Lysmatidae Common/FAO : Pacific cleaner Name (English) shrimp Local names:names Not available MORPHOLOGICAL DESCRIPTION The Pacific cleaner shrimp is easily identified by its colour patterns. The body is light brown with one white band dorsally and two red bands laterally running longitudinally. The tail has two white spots on either side. The antennae are white in colour and the first pair has red coloured base. It grows up to a maximum of 6 cm. Source of image : RC CMFRI, Vizhinjam 363 PROFILE GEOGRAPHICAL DISTRIBUTION Scarlet cleaner shrimp or Pacific cleaner shrimp is one of the most popular species of ornamental crustaceans distributed in the waters of the Indo-Pacific region in Indonesia and Sri Lanka. HABITAT AND BIOLOGY It is one of the popular marine shrimp, associated with coral reefs and compatible with smaller sized marine ornamental fishes. It hides in the near shore, shallow and protected areas within a temperature range of 25-30 °C. In the Indo-Pacific areas and the Red Sea, it is mostly found in caves and crevices of coral reefs. It especially needs shelter from predators when it is moulting. It is an omnivore and a scavenger and often feeds on the external parasites of fishes. As its name indicates, this species cleans fishes including moray eels and groupers feeding on their external parasites as well as on mucous and dead or injured tissue. The shrimp moults once every 3-8 weeks and spawns regularly every 2-3 weeks. -

Multiple Colonizations Lead to Cryptic Biodiversity in an Island Ecosystem: Comparative Phylogeography of Anchialine Shrimp Species in the Ryukyu Archipelago, Japan
Reference: Biol. Bull. 225: 24–41. (September 2013) © 2013 Marine Biological Laboratory Multiple Colonizations Lead to Cryptic Biodiversity in an Island Ecosystem: Comparative Phylogeography of Anchialine Shrimp Species in the Ryukyu Archipelago, Japan DAVID A. WEESE1,* YOSHIHISA FUJITA2,3, AND SCOTT R. SANTOS1,4 1Department of Biological Sciences and Molette Biology Laboratory for Environmental and Climate Change Studies, Auburn University, 101 Life Sciences Building, Auburn, Alabama 36849; 2University Education Center, University of the Ryukyus, 1 Senbaru, Nishihara-cho, Okinawa 903-0213, Japan; 3Marine Learning Center, 2-95-101 Miyagi, Chatan-cho, Okinawa 904-0113, Japan; 4Cell and Molecular Biosciences Peak Program, Auburn University, 101 Life Sciences Building, Auburn, Alabama 36849 Abstract. Archipelagos of the Indo-West Pacific are con- results, when interpreted in the context of Pacific oceano- sidered to be among the richest in the world in biodiversity, graphic currents and geologic history of the Ryukyus, imply and phylogeographic studies generally support either the multiple colonizations of the archipelago by the three spe- center of origin or the center of accumulation hypothesis to cies, consistent with the center of accumulation hypothesis. explain this pattern. To differentiate between these compet- While this study contributes toward understanding the bio- ing hypotheses for organisms from the Indo-West Pacific diversity, ecology, and evolution of organisms in the anchialine ecosystem, defined as coastal bodies of mixoha- Ryukyus and the Indo-West Pacific, it also has potential line water fluctuating with the tides but having no direct utility in establishing conservation strategies for anchialine oceanic connections, we investigated the genetic variation, fauna of the Pacific Basin in general. -

Understanding Transformative Forces of Aquaculture in the Marine Aquarium Trade
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Summer 8-22-2020 Senders, Receivers, and Spillover Dynamics: Understanding Transformative Forces of Aquaculture in the Marine Aquarium Trade Bryce Risley University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Marine Biology Commons Recommended Citation Risley, Bryce, "Senders, Receivers, and Spillover Dynamics: Understanding Transformative Forces of Aquaculture in the Marine Aquarium Trade" (2020). Electronic Theses and Dissertations. 3314. https://digitalcommons.library.umaine.edu/etd/3314 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. SENDERS, RECEIVERS, AND SPILLOVER DYNAMICS: UNDERSTANDING TRANSFORMATIVE FORCES OF AQUACULTURE IN THE MARINE AQUARIUM TRADE By Bryce Risley B.S. University of New Mexico, 2014 A THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Marine Policy and Marine Biology) The Graduate School The University of Maine May 2020 Advisory Committee: Joshua Stoll, Assistant Professor of Marine Policy, Co-advisor Nishad Jayasundara, Assistant Professor of Marine Biology, Co-advisor Aaron Strong, Assistant Professor of Environmental Studies (Hamilton College) Christine Beitl, Associate Professor of Anthropology Douglas Rasher, Senior Research Scientist of Marine Ecology (Bigelow Laboratory) Heather Hamlin, Associate Professor of Marine Biology No photograph in this thesis may be used in another work without written permission from the photographer. -

Biodiversity: the UK Overseas Territories. Peterborough, Joint Nature Conservation Committee
Biodiversity: the UK Overseas Territories Compiled by S. Oldfield Edited by D. Procter and L.V. Fleming ISBN: 1 86107 502 2 © Copyright Joint Nature Conservation Committee 1999 Illustrations and layout by Barry Larking Cover design Tracey Weeks Printed by CLE Citation. Procter, D., & Fleming, L.V., eds. 1999. Biodiversity: the UK Overseas Territories. Peterborough, Joint Nature Conservation Committee. Disclaimer: reference to legislation and convention texts in this document are correct to the best of our knowledge but must not be taken to infer definitive legal obligation. Cover photographs Front cover: Top right: Southern rockhopper penguin Eudyptes chrysocome chrysocome (Richard White/JNCC). The world’s largest concentrations of southern rockhopper penguin are found on the Falkland Islands. Centre left: Down Rope, Pitcairn Island, South Pacific (Deborah Procter/JNCC). The introduced rat population of Pitcairn Island has successfully been eradicated in a programme funded by the UK Government. Centre right: Male Anegada rock iguana Cyclura pinguis (Glen Gerber/FFI). The Anegada rock iguana has been the subject of a successful breeding and re-introduction programme funded by FCO and FFI in collaboration with the National Parks Trust of the British Virgin Islands. Back cover: Black-browed albatross Diomedea melanophris (Richard White/JNCC). Of the global breeding population of black-browed albatross, 80 % is found on the Falkland Islands and 10% on South Georgia. Background image on front and back cover: Shoal of fish (Charles Sheppard/Warwick -

Prawn Fauna (Crustacea: Decapoda) of India - an Annotated Checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean Prawns
Available online at: www.mbai.org.in doi: 10.6024/jmbai.2012.54.1.01697-08 Prawn fauna (Crustacea: Decapoda) of India - An annotated checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean prawns E. V. Radhakrishnan*1, V. D. Deshmukh2, G. Maheswarudu3, Jose Josileen 1, A. P. Dineshbabu4, K. K. Philipose5, P. T. Sarada6, S. Lakshmi Pillai1, K. N. Saleela7, Rekhadevi Chakraborty1, Gyanaranjan Dash8, C.K. Sajeev1, P. Thirumilu9, B. Sridhara4, Y Muniyappa4, A.D.Sawant2, Narayan G Vaidya5, R. Dias Johny2, J. B. Verma3, P.K.Baby1, C. Unnikrishnan7, 10 11 11 1 7 N. P. Ramachandran , A. Vairamani , A. Palanichamy , M. Radhakrishnan and B. Raju 1CMFRI HQ, Cochin, 2Mumbai RC of CMFRI, 3Visakhapatnam RC of CMFRI, 4Mangalore RC of CMFRI, 5Karwar RC of CMFRI, 6Tuticorin RC of CMFRI, 7Vizhinjam RC of CMFRI, 8Veraval RC of CMFRI, 9Madras RC of CMFRI, 10Calicut RC of CMFRI, 11Mandapam RC of CMFRI *Correspondence e-mail: [email protected] Received: 07 Sep 2011, Accepted: 15 Mar 2012, Published: 30 Apr 2012 Original Article Abstract Many penaeoid prawns are of considerable value for the fishing Introduction industry and aquaculture operations. The annual estimated average landing of prawns from the fishery in India was 3.98 The prawn fauna inhabiting the marine, estuarine and lakh tonnes (2008-10) of which 60% were contributed by freshwater ecosystems of India are diverse and fairly well penaeid prawns. An additional 1.5 lakh tonnes is produced from known. Significant contributions to systematics of marine aquaculture. During 2010-11, India exported US $ 2.8 billion worth marine products, of which shrimp contributed 3.09% in prawns of Indian region were that of Milne Edwards (1837), volume and 69.5% in value of the total export. -

Belgian Register of Marine Species
BELGIAN REGISTER OF MARINE SPECIES September 2010 Belgian Register of Marine Species – September 2010 BELGIAN REGISTER OF MARINE SPECIES, COMPILED AND VALIDATED BY THE VLIZ BELGIAN MARINE SPECIES CONSORTIUM VLIZ SPECIAL PUBLICATION 46 SUGGESTED CITATION Leen Vandepitte, Wim Decock & Jan Mees (eds) (2010). Belgian Register of Marine Species, compiled and validated by the VLIZ Belgian Marine Species Consortium. VLIZ Special Publication, 46. Vlaams Instituut voor de Zee (VLIZ): Oostende, Belgium. 78 pp. ISBN 978‐90‐812900‐8‐1. CONTACT INFORMATION Flanders Marine Institute – VLIZ InnovOcean site Wandelaarkaai 7 8400 Oostende Belgium Phone: ++32‐(0)59‐34 21 30 Fax: ++32‐(0)59‐34 21 31 E‐mail: [email protected] or [email protected] ‐ 2 ‐ Belgian Register of Marine Species – September 2010 Content Introduction ......................................................................................................................................... ‐ 5 ‐ Used terminology and definitions ....................................................................................................... ‐ 7 ‐ Belgian Register of Marine Species in numbers .................................................................................. ‐ 9 ‐ Belgian Register of Marine Species ................................................................................................... ‐ 12 ‐ BACTERIA ............................................................................................................................................. ‐ 12 ‐ PROTOZOA ........................................................................................................................................... -

Crangon Franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Crangon franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Caridea Common gray shrimp Family: Crangonoidea, Crangonidae Taxonomy: Schmitt (1921) described many duncle segment (Wicksten 2011). Inner fla- shrimp in the genus Crago (e.g. Crago fran- gellum of the first antenna is greater than ciscorum) and reserved the genus Crangon twice as long as the outer flagellum (Kuris et for the snapping shrimp (now in the genus al. 2007) (Fig. 2). Alpheus). In 1955–56, the International Mouthparts: The mouth of decapod Commission on Zoological Nomenclature crustaceans comprises six pairs of appendag- formally reserved the genus Crangon for the es including one pair of mandibles (on either sand shrimps only. Recent taxonomic de- side of the mouth), two pairs of maxillae and bate revolves around potential subgeneric three pairs of maxillipeds. The maxillae and designation for C. franciscorum (C. Neocran- maxillipeds attach posterior to the mouth and gon franciscorum, C. franciscorum francis- extend to cover the mandibles (Ruppert et al. corum) (Christoffersen 1988; Kuris and Carl- 2004). Third maxilliped setose and with exo- ton 1977; Butler 1980; Wicksten 2011). pod in C. franciscorum and C. alaskensis (Wicksten 2011). Description Carapace: Thin and smooth, with a Size: Average body length is 49 mm for single medial spine (compare to Lissocrangon males and 68 mm for females (Wicksten with no gastric spines). Also lateral (Schmitt 2011). 1921) (Fig. 1), hepatic, branchiostegal and Color: White, mottled with small black spots, pterygostomian spines (Wicksten 2011). giving gray appearance. Rostrum: Rostrum straight and up- General Morphology: The body of decapod turned (Crangon, Kuris and Carlton 1977).