Randall Kramer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Humanities I 2005
Humanities I 2005 FACULTIES OF THE UNIVERSITY OF PRETORIA HUMANITIES NATURAL AND AGRICULTURAL SCIENCES LAW THEOLOGY ECONOMIC AND MANAGEMENT SCIENCES VETERINARY SCIENCE EDUCATION HEALTH SCIENCES ENGINEERING, BUILT ENVIRONMENT AND INFORMATION TECHNOLOGY Address all correspondence to: The Director: Academic Administration University of Pretoria PRETORIA 0002 Cheques and postal orders must be crossed and made payable to the University of Pretoria. Telephone: 012 420 4111 Fax: 012 362 5168/362 5190 Web address: http://www.up.ac.za/ ISBN 1-86854-535-0 Humanities I 2005 FACULTY OF HUMANITIES PART I (this publication) SCHOOL OF LANGUAGES • Afrikaans • African Languages • Ancient Languages • Centre for Academic Development • English • Modern European Languages • Unit for Language Skills Development SCHOOL OF SOCIAL SCIENCES • Anthropology and Archaeology • Biokinetics, Sport and Leisure Sciences • Centre for Augmentative and Alternative Communication • Communication Pathology • Criminology • Geography, Geoinformatics and Meteorology • Historical and Heritage Studies • Philosophy • Political Sciences • Psychology • Social Work • Sociology SCHOOL OF THE ARTS • Drama • Music • Visual Arts PART II (separate publication) POSTGRADUATE STUDIES Humanities I 2005 TABLE OF CONTENTS ACADEMIC STAFF ...........................................................................................................1 REGULATIONS FOR UNDERGRADUATE STUDY ........................................................9 REQUIREMENTS FOR ADMISSION ...............................................................................9 -

RUTHIRA NARAIDOO, Ph.D
RUTHIRA NARAIDOO, Ph.D. Associate Professor Department of Economics University of Pretoria Pretoria 0002 South Africa Tel: +27 12 420 3729 Fax:+27 12 362 5207 Email: [email protected] Website: http://web.up.ac.za/default.asp?ipkCategoryID=729&subid=729&ipklookid=3 PROFESSIONAL EXPERIENCE 2012- Associate Professor in Economics Department of Economics, University of Pretoria, South Africa 2008-2011 Senior Lecturer in Economics Department of Economics, University of Pretoria, South Africa 2007-2008 Lecturer in Economics The University of Nottingham Ningbo, China 2005 -2007 Lecturer in Economics School of Economic and Management Studies, Keele University, UK 2004-2005 Research Assistant/Teaching Assistant (Graduate Assistant) Economics Department, Cardiff Business School, UK Research Associate: Julian Hodge Institute for Applied Macroeconomics, Cardiff Business School, UK 1999-2004 Graduate Teaching Assistant/Research Associate Economics Department, Cardiff Business School, UK Research Associate: Julian Hodge Institute for Applied Macroeconomics, Cardiff Business School, UK VISITING POSITIONS Sept-Oct 2014 Visiting Fellow, Julian Hodge Institute for Applied Macroeconomics, Cardiff Business School, UK EDUCATION 1999-2004 PhD in Economics Economics Department, Cardiff Business School, UK Advisors: Patrick Minford (principal) and Kent Matthews 1998-1999 MSc in International Economics, Banking and Finance (Distinction) Economics Department, Cardiff Business School, UK 1994-1997 BSc in Economics University of Mauritius, Mauritius RESEARCH INTERESTS • Primary: Applied Macroeconomic Theory/Policy, Time Series Econometrics, Monetary Economics • Secondary: Political Economics, International Macroeconomics, Labour Economics, Financial Economics TEACHING PG Courses Lecturer and Module Coordinator: Open Economy Macroeconomics (PhD Level) Time Series Econometrics (Masters Level); Lecturer: Macroeconomics (PhD Level); Lecturer and Module Coordinator: Macroeconomics (Honours Level). -

Download Download
Journal of International Education Research – Second Quarter 2013 Volume 9, Number 2 Restructuring And Mergers Of The South African Post-Apartheid Tertiary System (1994-2011): A Critical Analysis Nelda Mouton, Ph.D., North-West University, South Africa G. P. Louw, Ph.D., North-West University, South Africa G. L. Strydom, Ph.D., North-West University, South Africa ABSTRACT Socio-economic and vocational needs of communities, governments and individuals change over the years and these discourses served as a compass for restructuring of higher institutions in South Africa from 1994. Before 1994, the claim to legitimacy for government policies in higher education rested on meeting primarily the interests of the white minority. From 1996 onwards, the newly established government considered education a major vehicle of societal transformation. The main objective had been to focus on reducing inequality and fostering internationalisation. Therefore, the rationale for the restructuring of South African universities included a shift from science systems to global science networks. Various challenges are associated with restructuring and include access, diversity, equity and equality. Thus, the restructuring and mergers between former technikons and traditional universities were probably the most difficult to achieve in terms of establishing a common academic platform, as transitional conditions also had to be taken into account and had a twin logic: It was not only the legacy of apartheid that had to be overcome but the incorporation of South Africa into the globalised world was equally important as globalisation transforms the economic, political, social and environmental dimensions of countries and their place in the world. Initially, the post-apartheid higher education transformation started with the founding policy document on higher education, the Report of the National Commission on Higher Education and this report laid the foundation for the 1997 Education White Paper 3 on Higher Education in which a transformed higher education system is described. -

Annual Report 2019
ANNUAL REPORT 2019 ANNUAL REPORT FOR THE YEAR ENDED 31 DECEMBER 2019 TABLE OF CONTENTS I. ACRONYMS ........................................................................................................................................... 3 II. INSTITUTIONAL GOVERNANCE AND MANAGEMENT INFORMATION ............................................. 4 Organisational Structure ....................................................................................................................... 4 Rhodes University Governance & Management Organogram in 2019 ................................................ 4 1. ANNUAL PERFORMANCE ASSESSMENT REPORT OF THE APP ..................................................... 5 1.1 Background .................................................................................................................................. 5 2. REPORT BY THE CHAIRPERSON OF COUNCIL ................................................................................. 9 3. COUNCIL STATEMENT ON GOVERNANCE ...................................................................................... 10 3.1 Statutory Governance Information ............................................................................................. 10 3.2 Composition of Council .............................................................................................................. 11 3.3 Summary of Attendance at Meetings of Council and Committees of Council ............................ 11 3.4 Major Statements/decisions of council ...................................................................................... -

2021/06/01 Members of Rating Panels 2020/21 Panel Role Member Institution Department NRF Contact Person
2021/06/01 Members of Rating Panels 2020/21 Panel Role Member Institution Department NRF Contact Person Convener Adesina, JOT (Prof) University of South Africa Archie Mafeje Research Institute Member Aswani, SAC (Prof) Rhodes University Department of Anthropology Lizzy Ledwaba Member Mokomane, ZS (Prof) University of Pretoria Department of Sociology [email protected] 012 481 4089 Social Work Social Member Schoeman, MH (Dr) University of the Witwatersrand Archaeology Anthropology, Anthropology, Development Studies, Studies, Development School of Built Environment and Member Sutherland, CG (Dr) University of KwaZulu-Natal Geography, Sociology and and Geography, Sociology Development Studies Biochemistry, Genetics and Member Makhalanyane, TP (Prof) University of Pretoria Microbiology Microbial, Biochemical and Food Member Pohl-Albertyn, CH (Prof) University of the Free State Matshidisho Mabala Biotechnology [email protected] Convener Trindade, IM (Prof) University of the Western Cape Department of Biotechnology 012 481 4091 Microbiology Basic and Applied Applied and Basic Member Wolfaardt, GM (Prof) Stellenbosch University Microbiology Member Hammerbacher, AH (Dr) University of Pretoria Zoology and Entomology South African Medical Research Biomedical Research and Member Johnson, R (Dr) Council (MRC) Innovation Platform Wieneke Huizinga Co- [email protected] 012 Kana, BD (Prof) University of the Witwatersrand School of Pathology convener 481 481 4052 & Cell Biology Cell & Department of Parasites, Co- ARC, Onderstepoort Veterinary -

U.S. -‐ Universities Masters Phd Human Rights Arizona State
U.S. - Universities Masters PhD Human Rights Arizona State University X X City University of New York X Columbia Univeristy X Cornell University X Fordham University X New York University X Pace University X Syracuse University X University of Hawaii - Manoa X American University X Capella University X X Indiana University X X Marquette University In Process University of Denver X X University of Dayton X University of Massachusetts - Amherst X University of Cincinnati X Yale University X University of Washington X Boston College X Tufts University X University of Connecticut - Storrs X University of Minnesota X Trinity College X Emory University X University of Pennsylvania X Walden University X X University of San Francisco X Human Rights - Law Northwestern University X Emory University X Columbia University X University of Connecticut X University of Texas - Austin X Indiana University X University of Notre Dame X X American University X Harvard University X Georgetown University X X University of Pittsburgh X Depaul University X University of Minnesota X St. Thomas University X University of California - Los Angeles X University of Virginia X University of Miami X Drake University X Peace Studies American University X X Nova Southeastern University X Southern Methodist University X University of Denver X University of North Carolina - Greensboro X University of Notre Dame X X Salisbury University X George Mason University X X Ohio State University X University of San Diego X Duke University X University of North Carolina - Chapel -

Mlambo-Abridged-Cv.Zp65795.Pdf
UNIVERSITY OF PRETORIA FACULTY OF HUMANITIES ABRIDGED CURRICULUM VITAE Surname Mlambo First names Alois EDUCATION Degree/ Qualification Field of study Higher education institution Diploma Year BA (Hons.) English and History University of Canterbury, Kent, UK 1977 MA African History School of Oriental and African Studies, 1980 University of London, UK MA American History Wesleyan University, Connecticut, USA 1982 PhD Latin American History Duke University, North Carolina, USA 1989 WORK EXPERIENCE TO DATE Name of employer Capacity and/or type of work Period Bayero University, Kano, Nigeria Lecturer in History Jan-Aug 1980 University of Zimbabwe Lecturer - Associate Professor Jan. 1981- Mar. 2004 Duke University Visiting Lecturer Jan – May 1992/Jan-May 1995 University of North Carolina Visiting Lecturer Jan-May 1992 University of Minnesota Visiting Associate Professor Sept. – Dec. 1999 Cumberland Lodge, Windsor, The Great Park, UK The first Queen Elizabeth Fellow of the King George VI & Queen Elizabeth Foundation of St. Catherine [Funded to Sept. – Dec. 2002 organise an international conference on human rights] United States Coast Guard Senior Fulbright Fellow Jan – May 2002 Academy University of Pretoria Professor of History Since April 2004 to date 1 COURSES TAUGHT * The African experience in the Diaspora * Introduction to Economic History: Theory and Methodology * Social and Economic History of Latin America and the Caribbean * Social and Economic History of North America * Africa South of the Sahara * Introduction to African History -

Details of Authors
Details of authors Stephanie G Burton B Sc (Hons) Graduate Sue Harrison B Sc (Hons)(Microbiology) PhD Certificate in Education (University of Rhodesia) (Chemical Engineering) MSc PhD (Rhodes University) MASSAf, MSCI, FSAAE, FWISA, FSAIMM, Vice-Principal, University of Pretoria, Private Bag MSASM, MSAIChE, MIWA X20, Hatfield, Pretoria, 0028 Director of the Centre for Bioprocess Engineering Tel: +27 12 420-2970 Research, Department of Chemical Engineering, Fax: +27 12 420-3696 University of Cape Town, South Africa E-mail: [email protected] Tel: +27 21 650 4021 Professor Stephanie Burton holds an MSc in E-mail: [email protected] Organic Chemistry (1990) and a PhD in Prof. Sue Harrison holds the SARChI Research Biochemistry (1994) from Rhodes University. Chair in Bioprocess Engineering at the University of Professor Burton has lectured in Biochemistry and Cape Town. She is Deputy Dean (Postgraduate and Biotechnology at Rhodes University, and then Research) in the Faculty of Engineering and the Chemical Engineering at the University of Cape Built Environment, and Director of the Centre for Town. In 2009, she was appointed Director of Bioprocess Engineering Research (CeBER) in the Postgraduate Studies at Cape Peninsula University Department of Chemical Engineering at UCT. She of Technology. Professor Burton took up her pres- has over twenty years’ experience in industrial and ent position as Vice-Principal: Research and academic research, and in university teaching. This Postgraduate Education at the University of time has resulted in nearly 100 co-authored Pretoria, in 2011. Her research interests are in the research papers in refereed journals and books, 80 field of applied biochemistry and biotechnology, international conference presentations, and super- including biocatalysis, bioremediation and benefici- vision of almost 60 postgraduate students to com- ation of industrial wastes. -

African Studies November 2009 University of Michigan Iiiiii Center Alliances No.2 Member of the University of Michigan International Institute
African Studies November 2009 University of Michigan iiiiii Center alliances No.2 Member of the University of Michigan International Institute ASC Launch Conference a Great Success INTerNATIoNAL SyMPoSIUM feATUreS SCHoLArS, DIgNITArIeS iimidst much good cheer Aand palpable excitement, the African Studies Center marked its academic debut with an international symposium featuring an impressive array iiof national and international scholars and dignitaries. The March 12-14, 2009 event, “Engaging Africa/ Advancing African Studies,” fulfilled its dual peace continues to consolidate with each objectives of honoring the first anniversary passing year—even as victims’ rights of U-M President Mary Sue Coleman’s trip are ignored. He argued that the need for to Africa, and advancing the intellectual peace and reconciliation within families iiagenda for Africa initiatives at the U-M. evolved outward from the grassroots level These initiatives represent the ‘Michigan to the country as a whole. He then turned Difference’ by spanning health, science, to Kenya, where a fragile peace has set in humanities, engineering, public policy, law, following the election violence of January the arts, and more―the full gamut of the 2008, and argued for improved coordination university. among international actors assisting with Following remarks by Senior Vice Provost for Academic Affairs Lester Monts, Interna- tional Institute Director Mark Tessler, and ASC Director Kelly Askew, the symposium began with a keynote address on “Peace Without Justice” by João Honwana, Director of the Africa I Division/UN Department of Political Affairs. Honwana is a leading figure in African conflict resolution who fought in the Mozambican struggle for independence. He directed the Conventional Arms Branch of the UN Department for Disarmament Affairs and led peace-building efforts in Guinea-Bissau and Sudan before being Amos Sawyer, former President of Liberia appointed to his current position. -

28 October 2021 Online
2 July 2020 Global Leadership Forum 28 October 2021 Online 1 “Disruptor or innovator, campaigner or catalyst, revolution or evolution? How will you change the world?” This is the challenge set to students on Global Leadership Forums. The programme gives students a chance to stand up and take ownership of the leadership that will define them, their generation and the impact they will have on the world. Over 4,252 students from 56 universities have interacted with 100 senior leaders on Global Leadership Forums online across the world since May 2020. 4,252 56 100 students universities leaders Global Leadership Forums help students to: explore key challenges in the workplace and in society understand what it means to be a leader appreciate when and how to lead across boundaries. 2 Programme Impact Students are asked to complete an assessment on their learning and leadership development at the end of each Global Leadership Forum. Across all 8 programmes globally so far, students have scored an average of*: 5.41 5.18 5.18 5.37 out of 6 out of 6 out of 6 out of 6 When asked if When asked if When asked if When asked if the Global they have a they have a the Global Leadership better better Leadership Forum understanding understanding Forum was good was a valuable of what it means of how leaders value for their learning to be a leader as tackle key time experience a result of the challenges as a Global result of the Leadership Global Forum Leadership Forum *Students score on a range of 1–6 (from strongly disagree to strongly agree), our target is an average score of 5. -

Yeukai Angela Mlambo Arizona State University Mary Lou Fulton Teachers College Center for Advanced Studies in Global Education, 1000 S
Yeukai Angela Mlambo Arizona State University Mary Lou Fulton Teachers College Center for Advanced Studies in Global Education, 1000 S. Forest Mall, Payne Hall 203F Tempe, Arizona, 85287 Phone: (469)-200-9104 Email: [email protected] ______________________________________________________________________________ CITIZENSHIP - Citizen of the United States of America - Zimbabwean & permanent resident of South Africa ______________________________________________________________________________ EDUCATION Doctor of Philosophy (Ph.D.) MICHIGAN STATE UNIVERSITY, East Lansing, MI Educational Administration: Higher, Adult and Lifelong Education May 2017 Chair: Dr. Brendan Cantwell (Associate Professor) Master of Arts (M.A.) UNIVERSITY OF PRETORIA, South Africa Research Psychology April 2012 Chair: Prof. Cheryl de la Rey (Vice Chancellor, University of Pretoria) Bachelor of Social Sciences (BSoc. Sci. Honors) UNIVERSITY OF PRETORIA, South Africa Psychology April 2010 ______________________________________________________________________________ PROFESSIONAL AND RESEARCH EXPERIENCE Assistant Research Professor & Assistant Director for Research & Evaluation Center for Advanced Studies in Global Education Mary Lou Fulton Teachers College, Arizona State University, (August 2019 – to-date) • Leads data collection, analysis, knowledge mobilization and Monitoring, Evaluation and Learning for three projects funded by the MasterCard Foundation • Manages research related budget lines for all fieldwork activities. • Recruits, hires, trains and manages -
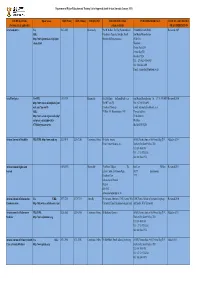
DHET Local Journals (2021)
Department of Higher Education and Training List of Approved South African Journals (January 2021) JOURNAL TITLE Open Access ISSN (Print) ISSN (Online) FREQUENCY EDITOR'S DETAILS PUBLISHER'S DETAILS DATE OF LAST REVIEW (Previous title if applicable) (when available) OR ACCREDITATION Acta Academica Yes 0587-2405 Bi-annually Prof H Melber, The Dag Hammarskjold PUBLISHER ADDRESS Reviewed 2009 URL: Foundation Uppsala, Sweden, Email: Sun Media Bloemfontein http://www.journals.co.za/ej/ejour_ [email protected] 55 Brill St classic.html Westdene Postnet Suite 214 Private Bag X01 Brandhof 9324 Tel : +27-(0)51-430-0459 Fax : 086-545-4491 E-mail : [email protected] Acta Theologica Yes URL: 1015-8758 Biannually Dr L Hoffman [email protected] Sun Media Bloemfontein Tel: + 27 51 430 0459 Reviewed 2010 http://www.ufs.ac.za/templates/jour Prof HC van Zyl Fax: +27 86 545 4491 nals.aspx?journal=5 Faculty of Theology E-mail: [email protected] URL: PO Box 339, Bloemfontein,9300 Physical address: http://www.scielo.org.za/scielo.php? 59 Brill Street script=sci_serial&pid=1015- Westdene 8758&lng=en&nrm=iso BLOEMFONTEIN African Journal of Disability YES, URL: http://www.ajod.org 2223-9170 2226-7220 Continuous Online Dr Leslie Swartz AOSIS, Postnet Suite #110, Private Bag X19, Added in 2014 Email: [email protected] Durbanville, South Africa, 7551 Tel: 086 1000 381 Tel: +27 21 975 2602 Fax: 086 5004 974 African Human Rights Law 1609-073X Biannually Prof Frans Viljoen The Juta Law PO Box Reviewed 2011 Journal Editor: Centre for Human Right 24299