Tampa Bay Benthic Monitoring Program Interpretive Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Marine and Brackish Water Mollusca of the State of Mississippi
Gulf and Caribbean Research Volume 1 Issue 1 January 1961 The Marine and Brackish Water Mollusca of the State of Mississippi Donald R. Moore Gulf Coast Research Laboratory Follow this and additional works at: https://aquila.usm.edu/gcr Recommended Citation Moore, D. R. 1961. The Marine and Brackish Water Mollusca of the State of Mississippi. Gulf Research Reports 1 (1): 1-58. Retrieved from https://aquila.usm.edu/gcr/vol1/iss1/1 DOI: https://doi.org/10.18785/grr.0101.01 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf Research Reports Volume 1, Number 1 Ocean Springs, Mississippi April, 1961 A JOURNAL DEVOTED PRIMARILY TO PUBLICATION OF THE DATA OF THE MARINE SCIENCES, CHIEFLY OF THE GULF OF MEXICO AND ADJACENT WATERS. GORDON GUNTER, Editor Published by the GULF COAST RESEARCH LABORATORY Ocean Springs, Mississippi SHAUGHNESSY PRINTING CO.. EILOXI, MISS. 0 U c x 41 f 4 21 3 a THE MARINE AND BRACKISH WATER MOLLUSCA of the STATE OF MISSISSIPPI Donald R. Moore GULF COAST RESEARCH LABORATORY and DEPARTMENT OF BIOLOGY, MISSISSIPPI SOUTHERN COLLEGE I -1- TABLE OF CONTENTS Introduction ............................................... Page 3 Historical Account ........................................ Page 3 Procedure of Work ....................................... Page 4 Description of the Mississippi Coast ....................... Page 5 The Physical Environment ................................ Page '7 List of Mississippi Marine and Brackish Water Mollusca . Page 11 Discussion of Species ...................................... Page 17 Supplementary Note ..................................... -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Journal of Marine Research, Sears Foundation For
The Journal of Marine Research is an online peer-reviewed journal that publishes original research on a broad array of topics in physical, biological, and chemical oceanography. In publication since 1937, it is one of the oldest journals in American marine science and occupies a unique niche within the ocean sciences, with a rich tradition and distinguished history as part of the Sears Foundation for Marine Research at Yale University. Past and current issues are available at journalofmarineresearch.org. Yale University provides access to these materials for educational and research purposes only. Copyright or other proprietary rights to content contained in this document may be held by individuals or entities other than, or in addition to, Yale University. You are solely responsible for determining the ownership of the copyright, and for obtaining permission for your intended use. Yale University makes no warranty that your distribution, reproduction, or other use of these materials will not infringe the rights of third parties. This work is licensed under the Creative Commons Attribution- NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Journal of Marine Research, Sears Foundation for Marine Research, Yale University PO Box 208118, New Haven, CT 06520-8118 USA (203) 432-3154 fax (203) 432-5872 [email protected] www.journalofmarineresearch.org Species densities of macrobenthos associated with seagrass: A field experimental study of predation by David K. Young1, Martin A. Buzas2, and Martha W. -

A Reassessment of the Benthic Macrofaunal Community and Sediment Quality Conditions in Clam Bayou, Pinellas County, Florida: 2008 Vs
A Reassessment of the Benthic Macrofaunal Community and Sediment Quality Conditions in Clam Bayou, Pinellas County, Florida: 2008 vs. 2016 David J. Karlen, Ph.D.*; Thomas L. Dix, Ph. D.; Barbara K. Goetting; Sara E. Markham; Kevin W. Campbell; Joette M. Jernigan Environmental Protection Commission of Hillsborough County Data Report prepared for: Florida Department of Environmental Protection & Tampa Bay Estuary Program January 2017 *Author contact: [email protected] i Acknowledgements The Pinellas County Public Works Department, Environmental Management Division staff collected the benthic samples and field data for this study. The PCDEM personnel involved with the field work were: Melissa Harrison, Robert McWilliams, Mark Flock, Peggy Morgan, Conor Petren, Robin Barnes, and Julie Vogel. Laboratory processing of the silt/clay and benthic macrofauna samples was done by the Environmental Protection Commission of Hillsborough County. Anthony Chacour, Julie Christian, Lyndsey Grossmann, Lauren Lamonica, and Kirsti Martinez (EPCHC lab staff) assisted in the sample sorting and data entry. Sample analysis for sediment contaminants was conducted by the EPCHC’s chemistry lab under the direction of Joe Barron. Lab personnel involved were Amanda Weronik (metals), Lukasz Talalaj (pesticides, PCBs and PAHs), Kevin McCarthy (TOC) and Dawn Jaspard (Data Management). Funding was provided by the Tampa Bay Estuary Program as part of the annual bay-wide Tampa Bay Benthic Monitoring Program. i Table of Contents Acknowledgements ....................................................................................................................................... -

Luís Manuel Zambujal Chícharo Assistente Da
LUÍS MANUEL ZAMBUJAL CHÍCHARO ASSISTENTE DA UNIDADE DE CIÊNCIAS E TECNOLOGIAS DOS RECURSOS AQUÁTICOS UNIVERSIDADE DO ALGARVE SISTEMÁTICA, ECOLOGIA E DINÂMICA DE LARVAS E PÓS-LARVAS DE BIVALVES NA RIA FORMOSA FARO 1996 TESES SD LUÍS MANUEL ZAMBUJAL CHÍCHARO ASSISTENTE DA UNIDADE DE CIÊNCIAS E TECNOLOGIAS DOS RECURSOS AQUÁTICOS UNIVERSIDADE DO ALGARVE SISTEMÁTICA, ECOLOGIA E DINÂMICA DE LARVAS E PÓS-LARVAS DE BIVALVES NA RIA FORMOSA Dissertação apresentada à Universidade do Algarve para obtenção do grau de Doutor em Ciências Biológicas, especialidade de Ecologia. FARO 1996 UNIVERSIDADE DO ALGARVE crpwir.O DE DOCUMENTAÇÃO Aos meus pais A benthic animal with a planktonic larval stage is a strange beast. Not only must it survive and prosper in two different realms, but it must also make the transitions to the plankton and back to the benthos. G.A. Jackson (1986) Agradecimentos Ao Professor Pedro Ré, que me acompanhou desde o trabalho de estágio sempre com a mesma disponibilidade e amizade, pela orientação. Ao Professor J. Pedro Andrade, pela orientação e pelo apoio sempre disponível durante as várias fases do trabalho. Ao Professor Martin Sprung pela ajuda na definição do plano de trabalho, bem como por ter ajudado na instalação da experiência, por ter colaborado na manutenção dos colectores e pela imensa quantidade de bibliografia que me facultou. Ao Professor Sadat Muzavor pelo empenho colocado na minha visita ao Institut Fur Meerkunde de Kiel. À Professora Lucília Sant Anna pelos esforços desenvolvidos com vista à obtenção de uma bolsa do Programa Erasmus para a minha estadia no Department of Zoology da Universidade de Abeerden. -

228100 N, Tomando El Camino De Los Conucos Hasta El Entronque
228100 N, tomando el camino de los Conucos hasta el entronque de Zapato en 101700 E, 231400 N, siguiendo por el camino de Zapato hasta el final en 100700 E, 231600 N, girando al N hasta el estero de Santa Catalina por el cual sale al mar en 100300 E, 234950 N, se continúa por la línea de costa hasta el nacimiento del estero Los Morros en 096800 E, 234900 N, el cual se dirige hasta el punto N de Barra Sorda en 094300 E, 235400 N, por la orilla NO de esta barra en su límite con los manglares hasta cruzar el camino de Faro Roncali al estero Palmarito en 091900 E, 231900 N, en dirección S a 150 m del terraplén del Sur hasta el Pantano de Caleta Larga en 092800 E, 229950 N, donde bordea el Pantano hasta 092600 E, 229850 N, siguiendo la línea recta virtual hasta 090800 E, 228750 N, punto inicial de este derrotero. • A los efectos de controlar adecuadamente las acciones que puedan repercutir negativamente sobre esta área protegida se establece una Zona de Amortiguamiento que comprende los 500 metros a partir del límite externo del área y que se indica en el Anexo Cartográfico. Anexo 3 Listado de especies de la flora Flora terrestre Flora marina División RHODOPHYTA Orden CORALLINALES Familia CORALLINACEA 1. Amphiroa beauvoisii Lamouroux 2. Amphiroa fragilissima (Linnaeus) Lamouroux 3. Amphiroa rigida Lamouroux 4. Haliptilon cubense (Montagne ex Kützing) Garbary et Johansen 5. Haliptilon subulatum (Ellis et Solander) Johansen 6. Hydrolithon farinosum (Lamouroux) Penrose et Chamberlain 7. Jania adhaerens Lamouroux 8. -
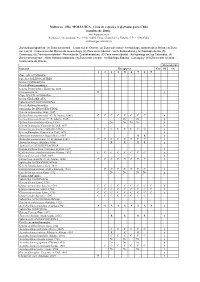
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Hermit Crabs - Paguridae and Diogenidae
Identification Guide to Marine Invertebrates of Texas by Brenda Bowling Texas Parks and Wildlife Department April 12, 2019 Version 4 Page 1 Marine Crabs of Texas Mole crab Yellow box crab Giant hermit Surf hermit Lepidopa benedicti Calappa sulcata Petrochirus diogenes Isocheles wurdemanni Family Albuneidae Family Calappidae Family Diogenidae Family Diogenidae Blue-spot hermit Thinstripe hermit Blue land crab Flecked box crab Paguristes hummi Clibanarius vittatus Cardisoma guanhumi Hepatus pudibundus Family Diogenidae Family Diogenidae Family Gecarcinidae Family Hepatidae Calico box crab Puerto Rican sand crab False arrow crab Pink purse crab Hepatus epheliticus Emerita portoricensis Metoporhaphis calcarata Persephona crinita Family Hepatidae Family Hippidae Family Inachidae Family Leucosiidae Mottled purse crab Stone crab Red-jointed fiddler crab Atlantic ghost crab Persephona mediterranea Menippe adina Uca minax Ocypode quadrata Family Leucosiidae Family Menippidae Family Ocypodidae Family Ocypodidae Mudflat fiddler crab Spined fiddler crab Longwrist hermit Flatclaw hermit Uca rapax Uca spinicarpa Pagurus longicarpus Pagurus pollicaris Family Ocypodidae Family Ocypodidae Family Paguridae Family Paguridae Dimpled hermit Brown banded hermit Flatback mud crab Estuarine mud crab Pagurus impressus Pagurus annulipes Eurypanopeus depressus Rithropanopeus harrisii Family Paguridae Family Paguridae Family Panopeidae Family Panopeidae Page 2 Smooth mud crab Gulf grassflat crab Oystershell mud crab Saltmarsh mud crab Hexapanopeus angustifrons Dyspanopeus -

Guide to Common Tidal Marsh Invertebrates of the Northeastern
- J Mississippi Alabama Sea Grant Consortium MASGP - 79 - 004 Guide to Common Tidal Marsh Invertebrates of the Northeastern Gulf of Mexico by Richard W. Heard University of South Alabama, Mobile, AL 36688 and Gulf Coast Research Laboratory, Ocean Springs, MS 39564* Illustrations by Linda B. Lutz This work is a result of research sponsored in part by the U.S. Department of Commerce, NOAA, Office of Sea Grant, under Grant Nos. 04-S-MOl-92, NA79AA-D-00049, and NASIAA-D-00050, by the Mississippi-Alabama Sea Gram Consortium, by the University of South Alabama, by the Gulf Coast Research Laboratory, and by the Marine Environmental Sciences Consortium. The U.S. Government is authorized to produce and distribute reprints for govern mental purposes notwithstanding any copyright notation that may appear hereon. • Present address. This Handbook is dedicated to WILL HOLMES friend and gentleman Copyright© 1982 by Mississippi-Alabama Sea Grant Consortium and R. W. Heard All rights reserved. No part of this book may be reproduced in any manner without permission from the author. CONTENTS PREFACE . ....... .... ......... .... Family Mysidae. .. .. .. .. .. 27 Order Tanaidacea (Tanaids) . ..... .. 28 INTRODUCTION ........................ Family Paratanaidae.. .. .. .. 29 SALTMARSH INVERTEBRATES. .. .. .. 3 Family Apseudidae . .. .. .. .. 30 Order Cumacea. .. .. .. .. 30 Phylum Cnidaria (=Coelenterata) .. .. .. .. 3 Family Nannasticidae. .. .. 31 Class Anthozoa. .. .. .. .. .. .. .. 3 Order Isopoda (Isopods) . .. .. .. 32 Family Edwardsiidae . .. .. .. .. 3 Family Anthuridae (Anthurids) . .. 32 Phylum Annelida (Annelids) . .. .. .. .. .. 3 Family Sphaeromidae (Sphaeromids) 32 Class Oligochaeta (Oligochaetes). .. .. .. 3 Family Munnidae . .. .. .. .. 34 Class Hirudinea (Leeches) . .. .. .. 4 Family Asellidae . .. .. .. .. 34 Class Polychaeta (polychaetes).. .. .. .. .. 4 Family Bopyridae . .. .. .. .. 35 Family Nereidae (Nereids). .. .. .. .. 4 Order Amphipoda (Amphipods) . ... 36 Family Pilargiidae (pilargiids). .. .. .. .. 6 Family Hyalidae . -

Ecological and Systematic Notes on Caecidae from St. Croix, U.S. Virgin
ECOLOGICAL AND SYSTEMATIC NOTES ON CAECIDAE FROM ST. CROIX, U. S. VIRGIN ISLANDS! DONALD R. MOORE University of Miami, Rosenstiel School of Marine and Atmospheric Science ABSTRACT A survey of the shallow marine fauna at St. Croix, U.S. Virgin Islands, was made in the summers of 1969 and 1970. Sediment samples were collected by diving, and micromollusks were picked from them. The Cae- cidae were studied from shallow-bay sediments and a quantitative study was made at three slightly deeper stations in and around the coral reefs. Seven species of Caecidae, 268 specimens, were found in 176 cc of sedi- ment from the three stations in deeper water. An eighth species was rare in the area of shallower water. The eight species, Caecum condylum Moore, C. subvolutum Folin, C. lineicinctum Folin, C. regulare Carpenter, C. textile Folin, C. imbricatum Carpenter, C. (Meioceras) nitidum Stimpson, and C. (M.) cornucopiae (Carpenter), are all poorly known. The first three species do not live in back-reef or lagoonal areas, and so were supposed to be extremely rare. However, these three species comprised 55 per cent of the Caecidae from the three stations in deeper water. They live in an environment that has been little sampled, so have been seldom collected. Distribution for all of the species is tropical, although C. nitidum and C. imbricatum are found in the northern Gulf of Mexico as well. INTRODUCTION Two brief surveys of the shallow marine environment of northeastern $1. Croix were undertaken by Dr. H. Gray Multer, Dr. Wayne D. Bock, and myself in 1969 and 1970. -

Benthic Invertebrate Species Richness & Diversity At
BBEENNTTHHIICC INVVEERTTEEBBRRAATTEE SPPEECCIIEESSRRIICCHHNNEESSSS && DDIIVVEERRSSIITTYYAATT DIIFFFFEERRENNTTHHAABBIITTAATTSS IINN TTHHEEGGRREEAATEERR CCHHAARRLLOOTTTTEE HAARRBBOORRSSYYSSTTEEMM Charlotte Harbor National Estuary Program 1926 Victoria Avenue Fort Myers, Florida 33901 March 2007 Mote Marine Laboratory Technical Report No. 1169 The Charlotte Harbor National Estuary Program is a partnership of citizens, elected officials, resource managers and commercial and recreational resource users working to improve the water quality and ecological integrity of the greater Charlotte Harbor watershed. A cooperative decision-making process is used within the program to address diverse resource management concerns in the 4,400 square mile study area. Many of these partners also financially support the Program, which, in turn, affords the Program opportunities to fund projects such as this. The entities that have financially supported the program include the following: U.S. Environmental Protection Agency Southwest Florida Water Management District South Florida Water Management District Florida Department of Environmental Protection Florida Coastal Zone Management Program Peace River/Manasota Regional Water Supply Authority Polk, Sarasota, Manatee, Lee, Charlotte, DeSoto and Hardee Counties Cities of Sanibel, Cape Coral, Fort Myers, Punta Gorda, North Port, Venice and Fort Myers Beach and the Southwest Florida Regional Planning Council. ACKNOWLEDGMENTS This document was prepared with support from the Charlotte Harbor National Estuary Program with supplemental support from Mote Marine Laboratory. The project was conducted through the Benthic Ecology Program of Mote's Center for Coastal Ecology. Mote staff project participants included: Principal Investigator James K. Culter; Field Biologists and Invertebrate Taxonomists, Jay R. Leverone, Debi Ingrao, Anamari Boyes, Bernadette Hohmann and Lucas Jennings; Data Management, Jay Sprinkel and Janet Gannon; Sediment Analysis, Jon Perry and Ari Nissanka. -

Noaa 13648 DS1.Pdf
r LOAI<CO Qpy N Guide to Gammon Tidal IVlarsh Invertebrates of the Northeastern Gulf of IVlexico by Richard W. Heard UniversityofSouth Alabama, Mobile, AL 36688 and CiulfCoast Research Laboratory, Ocean Springs, MS39564" Illustrations by rimed:tul""'"' ' "=tel' ""'Oo' OR" Iindu B. I utz URt,i',"::.:l'.'.;,',-'-.,":,':::.';..-'",r;»:.",'> i;."<l'IPUS Is,i<'<i":-' "l;~:», li I lb~'ab2 Thisv,ork isa resultofreseaich sponsored inpart by the U.S. Department ofCommerce, NOAA, Office ofSea Grant, underGrani Nos. 04 8 Mol 92,NA79AA D 00049,and NA81AA D 00050, bythe Mississippi Alabama SeaGrant Consortium, byche University ofSouth Alabama, bythe Gulf Coast Research Laboratory, andby the Marine EnvironmentalSciences Consortium. TheU.S. Government isauthorized toproduce anddistribute reprints forgovern- inentalpurposes notwithstanding anycopyright notation that may appear hereon. *Preseitt address. This Handbook is dedicated to WILL HOLMES friend and gentleman Copyright! 1982by Mississippi hlabama SeaGrant Consortium and R. W. Heard All rightsreserved. No part of thisbook may be reproduced in any manner without permissionfrom the author. Printed by Reinbold Lithographing& PrintingCo., BooneviBe,MS 38829. CONTENTS 27 PREFACE FamilyMysidae OrderTanaidacea Tanaids!,....... 28 INTRODUCTION FamilyParatanaidae........, .. 29 30 SALTMARSH INVERTEBRATES ., FamilyApseudidae,......,... Order Cumacea 30 PhylumCnidaria =Coelenterata!......, . FamilyNannasticidae......,... 31 32 Class Anthozoa OrderIsopoda Isopods! 32 Fainily Edwardsiidae. FamilyAnthuridae