New Information on Bostonia Perplexa an Unusual Member of the Calamopityaceae from North America
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Organisation of Palaeobotany IOP NEWSLETTER
INTERNATIONAL UNION OF BIOLOGIC A L S C IENC ES S ECTION FOR P A L A EOBOTANY International Organisation of Palaeobotany IOP NEWSLETTER 110 August 2016 CONTENTS FROM THE SECRETARY/TREASURER IPC XIV/IOPC X 2016 IOPC 2020 IOP MEMBERSHIP IOP EXECUTIVE COMMITTEE ELECTIONS IOP WEBMASTER POSITION WHAT HAPPENED TO THE OUPH COLLECTIONS? THE PALAEOBOTANY OF ITALY UPCOMING MEETINGS CALL FOR NEWS and NOTES The views expressed in the newsletter are those of its correspondents, and do not necessarily reflect the policy of IOP. Please send us your contributions for the next edition of our newsletter (June 2016) by M ay 30th, 2016. President: Johanna Eder-Kovar (G ermany) Vice Presidents: Bob Spicer (Great Britain), Harufumi Nishida (Japan), M ihai Popa (Romania) M embers at Large: Jun W ang (China), Hans Kerp (Germany), Alexej Herman (Russia) Secretary/Treasurer/Newsletter editor: M ike Dunn (USA) Conference/Congress Chair: Francisco de Assis Ribeiro dos Santos IOP Logo: The evolution of plant architecture (© by A. R. Hemsley) I OP 110 2 August 2016 FROM THE In addition, please send any issues that you think need to be addressed at the Business SECRETARY/TREASURER meeting. I will add those to the Agenda. Dear IOP Members, Respectfully, Mike I am happy to report, that IOP seems to be on track and ready for a new Executive Council to take over. The elections are IPC XIV/IOPC X 2016 progressing nicely and I will report the results in the September/October Newsletter. The one area that is still problematic is the webmaster position. We really to talk amongst ourselves, and find someone who is willing and able to do the job. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -

S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Structure, Development and Reproduction in Flowering Plants
Structure, Development and Reproduction in Flowering Plants Body Plan and Diversity in Form S.V.S Chauhan Professor Department of Botany B.R. Ambedkar University Khandari Campus Agra – 282002 [email protected] 1 Body Plan and Diversity in Form Every living organism has a fixed form and it is because of this reason that we are able to distinguish most of them just due to their external structure. Study of external morphology or external appearance of higher plants is necessary to describe the plants in an accurate fashion and to distinguish between almost similar looking plants. Therefore, the plants are identified by their morphological characters. Variation in plants is found not only in external forms but also in their anatomical characters which are represented by different types of tissue systems . Morphology along with anatomy constitute the base of studying pattern of life forms. Life Span of Plants On the basis of life span, plants are of three types: annuals, biennials and perennials. a) Annuals: These plants complete their life-cycle in a single growing season which varies from a few weeks to a few months. They pass the unfavourable period in the form of seeds. Examples are wheat, pea and sunflower, etc. b) Biennials: These plants complete their life-cycle in two growing seasons. In the first season; they grow only vegetatively and store food generally in the roots. In the second season, these plants grow at the expense of the stored food and form the flowering shoot bearing flowers, fruits and seeds. Then the plants die. radish, turnip, cabbage, etc. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

A New Microsporangiate Organ from the Lower Carboniferous of the Novgorod Region, Russia O
ISSN 0031-0301, Paleontological Journal, 2009, Vol. 43, No. 10, pp. 1316–1329. © Pleiades Publishing, Ltd., 2009. A New Microsporangiate Organ from the Lower Carboniferous of the Novgorod Region, Russia O. A. Orlovaa, N. R. Meyer-Melikian†,a, and N. E. Zavialovab a Moscow State University (MGU), Leninskie gory 1, Moscow, 119991 Russia b Borissiak Paleontological Institute of the Russian Academy of Sciences, ul. Profsoyuznaya 123, Moscow, 117997 Russia e-mail: [email protected] Received February 5, 2008 Abstract—A new species of the genus Telangiopsis, T. nonnae O. Orlova et Zavialova, was described on the basis of a microsporangiate organ from the Lower Carboniferous deposits of the Novgorod Region. The mor- phology of branching fertile axes, synangia, and sporangia was thoroughly studied. The three-dimensional sys- tem of fertile axes branches monopodially; ultimate axes bear numerous connivent bunches of synangia, which consist of three to six basally fused elongated ovate sporangia. The morphology and ultrastructure of prepollen grains were studied, which were extracted from the rock matrix surrounding the sporangia. The two-layered exine includes a well-developed endexine and an alveolate ectexine, with one–three rows of large thin-walled alveolae. The new species was compared with other Early Carboniferous microsporangiate organs. Key words: Early Carboniferous, Novgorod Region, fertile axis, synangia, Lyginopteridales, trilete prepollen, exine ultrastructure. DOI: 10.1134/S003103010910013X INTRODUCTION synangia and numerous casts and imprints of detached synangia were found in yellow ferruginous sandstone. During the three last decades, the interest of bota- In addition to the fertile axes, sterile remains of Lygi- nists dealing with fossil and modern plants to Carbon- nopteridales, Medullosales, and Calamopytiales were iferous synangiate pollen organs has considerably found (Orlova and Snigirevskii, 2003, 2004). -

THE EVOLUTION of XYLEM ANATOMY in EARLY TRACHEOPHYTES by ELISABETH ANNE BERGMAN
Conquering the terrestrial environment: the evolution of xylem anatomy in early tracheophytes Item Type text; Electronic Thesis Authors Bergman, Elisabeth Anne Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 27/09/2021 03:01:29 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/626731 CONQUERING THE TERRESTRIAL ENVIRONMENT: THE EVOLUTION OF XYLEM ANATOMY IN EARLY TRACHEOPHYTES By ELISABETH ANNE BERGMAN ____________________ A Thesis Submitted to The Honors College In Partial Fulfillment of the Bachelors Degree With Honors in Biology with an Emphasis in Biomedical Sciences THE UNIVERSITY OF ARIZONA D E C E M B E R 2 0 1 7 Approved by: ____________________________ Dr. Brian Enquist Department of Ecology and Evolutionary Biology Acknowledgements Many thanks go to all of those who made contributions, big and small, to my honors thesis, and more notably, my education. Foremost, I thank Dr. Brian Enquist for accepting me into his lab and serving as my mentor for two years. I appreciate all of the time he put in to meet with me and help me to develop my honors thesis. Additional thanks go to Dr. Sean Michaletz who first introduced me to the work that would eventually become my honors thesis. From the University of Santa Cruz, California, I thank Dr. -

Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga
Subject BOTANY Paper No V Paper Code BOT521 Topic Taxonomy and Diversity of Seed Plant: Gymnosperms & Angiosperms Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants UNIT- I BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants Classification of Gymnosperms. # Robert Brown (1827) for the first time recognized Gymnosperm as a group distinct from angiosperm due to the presence of naked ovules. BENTHAM and HOOKSER (1862-1883) consider them equivalent to dicotyledons and monocotyledons and placed between these two groups of angiosperm. They recognized three classes of gymnosperm, Cyacadaceae, coniferac and gnetaceae. Later ENGLER (1889) created a group Gnikgoales to accommodate the genus giankgo. Van Tieghem (1898) treated Gymnosperm as one of the two subdivision of spermatophyte. To accommodate the fossil members three more classes- Pteridospermae, Cordaitales, and Bennettitales where created. Coulter and chamberlain (1919), Engler and Prantl (1926), Rendle (1926) and other considered Gymnosperm as a division of spermatophyta, Phanerogamia or Embryoptyta and they further divided them into seven orders: - i) Cycadofilicales ii) Cycadales iii) Bennettitales iv) Ginkgoales v) Coniferales vi) Corditales vii) Gnetales On the basis of wood structure steward (1919) divided Gymnosperm into two classes: - i) Manoxylic ii) Pycnoxylic The various classification of Gymnosperm proposed by various workers are as follows: - i) Sahni (1920): - He recognized two sub-divison in gymnosperm: - a) Phylospermae b) Stachyospermae BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants ii) Classification proposed by chamber lain (1934): - He divided Gymnosperm into two divisions: - a) Cycadophyta b) Coniterophyta iii) Classification proposed by Tippo (1942):- He considered Gymnosperm as a class of the sub- phylum pteropsida and divided them into two sub classes:- a) Cycadophyta b) Coniferophyta iv) D. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -
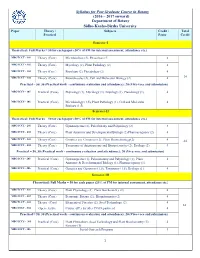
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

The Upper Carboniferous-Lower Permian Flora of Zöbing, Lower Austria
The upper Carboniferous-Lower Permian flora of Zöbing, Lower Austria Utrecht University Master Thesis Earth, Life and Climate By Koen Paalman 3470423 [email protected] 1 Contents page Abstract 3 1. Introduction 4 2. Geography, Geology and Lithology 6 3. Vegetation types 8 4. Different taxonomic groups 8 4.1 Calamites and other sphenopsids 8 4.2 Tree ferns 8 4.3 Cordaites 8 4.4 Pteridosperms 9 4.5 The medullosan pteridosperms 9 4.6 The peltaspermalean pteridosperms 9 5. Methods 10 6. Results 10 7. Correlation, comparison and interpretation 11 8. Discussion and conclusion 13 9. Acknowledgements 13 10. Appendix 14 10.1 Species list 14 10.2 Reference 16 11. Plates 19 2 Abstract During the Late Carboniferous and early Permian, a major floristic change took place in Euramerica. Gymnosperms replaced the previously dominant pteridophytes. This reflects a climatic change, i.e. from wetland-dominated to more arid conditions. Extensive studies on the vegetation during this time interval have recently been carried out in the Czech Republic. The Zöbing formation in Austria, is of the same age of these Czech formations, but has not yet been compared to them. Material from the Zöbing formation has been examined and compared to Czech floras. A clear transition can be seen in the flora of the Zöbing formation, from a Stephanian tree fern and Alethopteris zeilleri dominated flora, to a Asselian flora dominated by peltasperms and conifers. There are clear similarities between the floras of the Zöbing formation and the different Czechian formations, despite notable differences in species composition and abundance between Zöbing and the Czech formations. -

Curriculum Vitae
CURRICULUM VITAE ORCID ID: 0000-0003-0186-6546 Gar W. Rothwell Edwin and Ruth Kennedy Distinguished Professor Emeritus Department of Environmental and Plant Biology Porter Hall 401E T: 740 593 1129 Ohio University F: 740 593 1130 Athens, OH 45701 E: [email protected] also Courtesy Professor Department of Botany and PlantPathology Oregon State University T: 541 737- 5252 Corvallis, OR 97331 E: [email protected] Education Ph.D.,1973 University of Alberta (Botany) M.S., 1969 University of Illinois, Chicago (Biology) B.A., 1966 Central Washington University (Biology) Academic Awards and Honors 2018 International Organisation of Palaeobotany lifetime Honorary Membership 2014 Fellow of the Paleontological Society 2009 Distinguished Fellow of the Botanical Society of America 2004 Ohio University Distinguished Professor 2002 Michael A. Cichan Award, Botanical Society of America 1999-2004 Ohio University Presidential Research Scholar in Biomedical and Life Sciences 1993 Edgar T. Wherry Award, Botanical Society of America 1991-1992 Outstanding Graduate Faculty Award, Ohio University 1982-1983 Chairman, Paleobotanical Section, Botanical Society of America 1972-1973 University of Alberta Dissertation Fellow 1971 Paleobotanical (Isabel Cookson) Award, Botanical Society of America Positions Held 2011-present Courtesy Professor of Botany and Plant Pathology, Oregon State University 2008-2009 Visiting Senior Researcher, University of Alberta 2004-present Edwin and Ruth Kennedy Distinguished Professor of Environmental and Plant Biology, Ohio