Local Geology Controlled the Feasibility of Vitrifying Iron Age Buildings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hand-Made Pottery in the Prehistoric and Roman Period in Northern England and Southern Scotland
Durham E-Theses Native Pottery: hand-made pottery in the prehistoric and Roman period in northern England and southern Scotland Plowright, Georgina How to cite: Plowright, Georgina (1978) Native Pottery: hand-made pottery in the prehistoric and Roman period in northern England and southern Scotland, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/9824/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 NATIVE POTTERY: HAND-MADE POTTERY IN THE PREHISTORIC AND ROMAN PERIOD IN NORTHERN ENGLAND AND SOUTHERN SCOTLAND 'Thesis presented for the Degree of M.A. University of Durham Georgina Plowright October 1978 The copyright of this thesis rests with the author. No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. Abstract The thesis is a survey and catalogue of most of the pottery found in the area between the Clyde and Solway and the southern boundaries of Cumbria and Durham, and to which previously the label of Iron Age or Roman native pottery had been assigned. -

New Light on Oblong Forts: Excavations at Dunnideer, Aberdeenshire
Proc Soc Antiq Scot NEW140 (2010), LIGHT 79–91 ON OBLONG FORTS: EXCAVATIONS AT DUNNIDEER, ABERDEENSHIRE | 79 New light on oblong forts: excavations at Dunnideer, Aberdeenshire Murray Cook* with contributions by Hana Kdolska, Lindsay Dunbar, Rob Engl, Stefan Sagrott, Denise Druce and Gordon Cook ABSTRACT This paper presents the results of the excavation of a single keyhole trench at the oblong vitrified fort of Dunnideer, Aberdeenshire, along with a brief history of the study of oblong forts and vitrification. The excavation yielded two radiocarbon dates derived from destruction layers, which are discussed along with the results of a limited programme of archaeomagnetic dating at the same location. INTRODUCTION ramparts, it also has an entrance so may not be part of the oblong fort series. In addition, The series of oblong, gateless and often there has been significant debate over vitrified forts are one of the iconic type-sites of contradictory sets of dating evidence from the the Scottish Iron Age. Their study echoes the series (Alexander 2002). This article presents development of modern Scottish archaeology, the results and implications of the first new with its origins in the intellectual explosion excavation evidence for over 30 years. of the Scottish enlightenment; indeed, the earliest research (Williams 1777) just predates the founding of the Society of Antiquaries ARCHAEOLOGICAL BACKGROUND of Scotland in 1781. However, despite over 200 years of study, their function and date The forts in question are rectangular, with remain uncertain. This is largely because only massive stone timber-laced ramparts, two examples have been subject to modern frequently vitrified, without obvious excavation: Finavon, Tayside (MacKie 1969a entrances, often on prominent hilltops, and and 1976) and Craig Phadrig, Inverness ranging widely in area by a factor of 10 from (Small & Cottam 1972). -
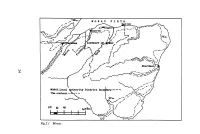
Man in Moray
10 0 I w! Fig.2.1 Moray. MANIN MORAY 5,000 years of history Ian Keillar Synopsis The extent of Moray is defined and the physical conditions briefly described. Traces of Mesolithic man have been found in the Culbin, and later Neolithic peoples found Moray an attractive place to settle. As metal working became established, trades routes followed and Moray flourished. As the climate deteriorated, so, apparently, did the political situation and defensive sites became necessary. The Romans came and went and the Picts rose and fell. The Vikings did not linger on these shores and MacBeth never met any witches near Forres. The Kings of Scots divided and ruled until they themselves set a pattern, which still continues, that if you want to get on you must go south to London. In distant Moray, brave men like Montrose and foolish men like Prince Charles Edward, fought for their rightful king. The Stuarts, however, ill rewarded their followers. Road makers and bridge builders half tamed the rivers, and the railways com pleted the process. With wars came boom years for the farmers, but even feather beds wear out and Moray is once more in apparent decline. However, all declines are relative and the old adage still has relevance: 'Speak wee] o the Hielans but live in the Laich.' Physical The name Moray is now applied to a local authority administrative District extending from west of Forres and the Findhorn to Cullen and stretching down in an irregular triangle into the highlands of the Cairngorms (Fig.2. l ). In Medieval times, Moray reached as far as Lochalsh on the west coast and there has always been some difficulty in defining the bound aries of the province. -

Iron Age Scotland: Scarf Panel Report
Iron Age Scotland: ScARF Panel Report Images ©as noted in the text ScARF Summary Iron Age Panel Document September 2012 Iron Age Scotland: ScARF Panel Report Summary Iron Age Panel Report Fraser Hunter & Martin Carruthers (editors) With panel member contributions from Derek Alexander, Dave Cowley, Julia Cussans, Mairi Davies, Andrew Dunwell, Martin Goldberg, Strat Halliday, and Tessa Poller For contributions, images, feedback, critical comment and participation at workshops: Ian Armit, Julie Bond, David Breeze, Lindsey Büster, Ewan Campbell, Graeme Cavers, Anne Clarke, David Clarke, Murray Cook, Gemma Cruickshanks, John Cruse, Steve Dockrill, Jane Downes, Noel Fojut, Simon Gilmour, Dawn Gooney, Mark Hall, Dennis Harding, John Lawson, Stephanie Leith, Euan MacKie, Rod McCullagh, Dawn McLaren, Ann MacSween, Roger Mercer, Paul Murtagh, Brendan O’Connor, Rachel Pope, Rachel Reader, Tanja Romankiewicz, Daniel Sahlen, Niall Sharples, Gary Stratton, Richard Tipping, and Val Turner ii Iron Age Scotland: ScARF Panel Report Executive Summary Why research Iron Age Scotland? The Scottish Iron Age provides rich data of international quality to link into broader, European-wide research questions, such as that from wetlands and the well-preserved and deeply-stratified settlement sites of the Atlantic zone, from crannog sites and from burnt-down buildings. The nature of domestic architecture, the movement of people and resources, the spread of ideas and the impact of Rome are examples of topics that can be explored using Scottish evidence. The period is therefore important for understanding later prehistoric society, both in Scotland and across Europe. There is a long tradition of research on which to build, stretching back to antiquarian work, which represents a considerable archival resource. -

Roman Influence on the Culture of Scotland
ROMAN INFLUENCE ON THE CULTURE OF SCOTLAND ENCOUNTERING AMERICA U). Rc^e -WARREN' STILSON 1 DECEMBER 1974 CONTENTS page I. I nt rorluct inn 1 II. Prehistoric limes 2 A. Mesolithic 2 B. Neolithic in 1.Western Scotland 3 2,Eastern Scotland 4 C. Bronze-Age 6 D. Iron-Age and the Coming of the Celts 8 1.Urnfield 2.Hallstatt I & II 9 3.La Tene 10 4.Gallic and Broch People 11 E. The Picts 15 III. The Roman Age 20 A. The Cause for the Initial Invasion of Scotland 21 B. ThR Fall of Rome in Britain 23 C. Neuu Kingdoms Established 24 D. The Spread of Christianity 26 IV. The Formation of the Kingdom of Scotland 28 A. The Growth into Political Unity 1.Peoples of the Kingdom 28 2.Wore on the Picts(As an Example) 29 B. Political Unity Achieved 31 V. Conclusion A. ulhat the Roman Empire Did Not 32 B. UJhat the Roman Empire Did 33 I.The Wost Significant Contribution 33 C. The Result 34 t/I. Notes 35 VII.Bibliography 36 • I. INTRIIOUCTION In the course of researching the culture of uihat is now the country of Scotland I mas assaulted by an inordinate number of maybe's anri possibly's, a few theories, and a few less facts. It seems that up to this day not enough archeological evidence has come to light to support the theories which are the primary invocation for this paper. It is through the three sciences of History, Philology, and Archeology from uihich all the evidence available has beenr accumulated. -

Inhaltsübersicht
Inhaltsübersicht. Erster Teil. I. Buch. Urbefestigungen. Seite Seite Einleitung 3—7 Crannoges 25 Befestigung mittels des Waldes Holzinsel im Drumaleague-See. 25. Burgwälle in Mecklenburg. 26. (Holzes) . 8-20 Ziegelinseln im Gebiet der Seille. 27. Hagen und Gebück 8 Sumpfburgen der Wetterau. 27. Grenzwehren der Servier u. Menapier.9. Wasserhügel (angebliche Das Fraxinetum der Sarazenen. 9. Hagedike der Normannen. 10. Mottes) '. 28 Schlesische Preseka. 10. Calosenkippel. 28. Böhmischer Gtrenzwald. 11. Klausenkippel. 29. Hackelwerke der alten Preussen. 11. Gewanenkippel. 30. Das Bheingauer Gebück. 12 — 15. Mederdorfelde. 30.' 'Andere Bezeichnungen der Baum- Hunsrücker Wasserburgen. 30. und Strauchbefestigungen. 15. Laudert. 31. Jagdhagen. 16. Dudenroth. 32. Knicke 16 Backer Schloss bei Torgau. 32. Verhaue 17 Anhang: Künstliche Hügel Wald- und Schlepp verhau. 17. auf trockenem Lande . 33 Verhau z. Sicherung d. Marsches. 17. Pfahlgrabentürme. 34. Ergänzungsverhaue. 18. Motte der Tapete von Bayeux. 34. Thorverschluss. 18. Spitzwälle in Nordostdeutschland. 34. Palisaden 18 Ausschaubügel am Mittelrhein. 34. Planken, Stakaden, Sturmpfähle Befestigung mit Steinen . 35—69 und Federbäume 19 Wallburgen 36 Römische Holzverschanzung 20 Ringwälle und Abschnittswälle. 36. Bauzeit und Bauart. 36. Befestigung mittels d. Wassers 21—34 . Ring von Otzenhausen. 38. Pfahlbauten 21 Bürgel bei Cronberg. 39. Schweizerische Pfahlbauten. 21. Odilienberg. 40. Mecklenburgische und Pommerische Kleine Felsringburgen. 44. Pfahlbauten. 22. Lurlei, Staufen, Rentmauer. 44. Muggenburg. 22. Rippenweyer Schanze. 44. Persanzig. 13. Cyklopenmauern. 44. Frankfurter Landhäuser. 14. Stickung. 45. http://d-nb.info/365455768 XL INHALTSÜBERSICHT. Seite Seite Gallische Mauern 46 Weitere Erklärungen. 65. Avaricum. 46. Engelburg. 65. Bibrakte. 47. Schluss. 66. Mursens und Luzech. 47. Wasserversorgung der Wall- ' Uxellodunum. 48. burgen 67 Gergovia. 49. Almerskopf. 67. -

Archaeological Measured Survey on Scotland’S National Forest Estate 2 | Archaeological Measured Survey Contents
Archaeological Measured Survey on Scotland’s national forest estate 2 | Archaeological Measured Survey contents A window onto the past 4 How archaeological measured survey is helping to support the conservation, investigation and interpretation of some of Scotland’s most signifi cant archaeological sites. Na Clachan Aoraidh 6 An unusual early Bronze Age stone circle above Loch Tummel. Ord Hill 8 A complex prehistoric landscape above Lairg. Dun Deardail 10 An Iron Age vitrifi ed fort above Glen Nevis. Caisteal Grugaig 12 An Iron Age broch on the headland of Totaig. Skelbo 14 A broch and outworks in Sutherland. Cracknie 16 A well-preserved Iron Age souterrain in Sutherland. Dun Boredale 18 A well-preserved Iron Age galleried dun on the Isle of Raasay. Carn Mor 20 A dun on the Black Isle. Rubha an Fhaing Dhuibh 22 A later prehistoric dun on Loch Shiel. Moat Park 24 A 12th century motte in Galloway. Nether Horsburgh 26 A 16th century tower house in the Scottish Borders. Leitir Fura 28 A cleared township on the south-east coast of Skye. Loch Arklet 30 The 18th century military road network on the national forest estate in the Highlands. Wester Drumclair 32 An 18th century farmstead in Limerigg Wood. Culbin Sands 34 The WWII anti-glider defences at Culbin. Wilsontown 36 An 18th century industrial landscape on the banks of the Mousewater in Lanarkshire. Woodmuir 38 Late 19th century coke ovens in West Lothian. Lossie 40 WWII coastal defences along the Moray coastline. Archaeological Measured Survey | 3 A window onto the past From Mesolithic flint scatters to the coastal defences of the Second World War, the archaeology on Scotland’s national forest estate spans thousands of years of history. -

By S. HIBBERT, M. D. F. R. S. Ed. &C. &C
Observations on Vitrified Forts. 161 of these forts had a volcanic origin ; and hence that volcanoes at some remote X.—Observations on the Theories which have been proposed to explain period had been very common in Scotland. This opinion was embraced by Mr Pennant, the eminent naturalist, who was led to it from an examination the Nitrified Forts of Scotland. of the Hill of Craig Phsedrick. A similar view was taken up by Thomas West, Esq. the author of a paper published in the Transactions of the Royal Society By S. HIBBERT, M. D. F. R. S. Ed. &c. &c. of London for the year 1777; and, four years afterwards, by the Hon. Daines Barrington. \_Read March 28/fc 1825.] The circumstances which led to this opinion have not been ill explained by Dr James Anderson of Monkshill, Aberdeenshire, in a letter dated the 27th THE members of this Society are no doubt familiar with what is meant by November 1777, and read to the Antiquarian Society of. London. " It must a Vitrified Fort. By this term is implied an area of ground, often of a round be owned," says this author, " that the natural appearance of the places where or elliptical form, and evidently selected for some natural defence possessed by these vitrified masses are usually found, is well calculated to favour the opi- it, which is farther protected by one or more inclosing ramparts, formed by nion that they have been produced by volcanoes. The vitrifiable matter is stones; these stones showing, to a greater or less extent, marks of vitrification, usually first discovered by travellers around the bottom and on the sides of steep by which they are cemented together. -

1971 Edition Have the Success and High Reputation of Its Predecessors
DISCOVERY EXCMV^ITI in SCOTLAND 19J1 Published by THE SCOTTISH REGIONAL GROUP Council for British Archaeology Price 25 new pence SCOTTISH REGIONAL GROUP COUNCIL FOR BRITISH ARCHAEOLOGY Hon. Secretary, c/o National Museum of Antiquities, Queen Street, Edinburgh EH2 1JD Membership of the Scottish Regional Group is open to archaeological and historical societies and to museums throughout Scotland. The Group was formed in 1944 to co-ordinate research on Scottish antiquities, to provide Scottish representation on the Council for British Archaeology and to further the cause of archaeology in Scotland. " Discovery and Excavation in Scotland " has been published annually by the Scottish Regional Group since 1956. Its purpose is to list by counties all discoveries which have taken place in Scotland over the past twelve months. Copies may be ordered from the Hon. Treasurer, c/o National Museum of Antiquities, Queen Street, Edinburgh. To be accepted for publication, contributions must conform to the standard format adopted by the Editorial Board. Potential contributors may obtain a copy of the appropriate instructions from the Hon. Secretary, c/o National Museum of Antiquities, Queen Street, Edinburgh. Contributions should be sent to :— Hon. Editor: Dr Margaret E. C Stewart, F.S.A.Scot., Tempar, 4 Dupplin Terrace, Kinnoall, Perth. SCOTTISH REGIONAL GROUP COUNCIL FOR BRITISH ARCHAEOLOGY Foreword It is my pleasure and privilege once again to launch a new issue of Discovery and Excavation in Scotland. May this 1971 edition have the success and high reputation of its predecessors. Despite constant rises in the cost of printing, the book is still modestly priced. The low cost of pro- duction is made possible by the voluntary work of the Joint Editors, Dr M. -

The History of Fettercairn, a Parish in the County of Kincardine
941.26019 F421C 1333860 GENEALOGY COLLECTION fu'll^l^iSnyf^.T,/. PUBLIC LIBRARY 3 1833 00859 0298 Digitized by the Internet Archive in 2010 with funding from Allen County Public Library Genealogy Center http://www.archive.org/details/historyoffettercOOcame » THE HISTORY OF FETTERCAIRN ft k THE HISTORY • OF FETTERCAIRX 1333860 |0 jfrteubs in jFettercatrn AND ©It) pupils NOW WIDELY SCATTERED, AND TO THE /Iftemor^ ot ^vicnt^s anC» pupils NOW DEPARTED, THESE PAGES ARt GRATEFULLY AND RESPECTFULLY PREFACE. The writing of this History of Fettercairn was first sug- gested to the author in 1882, after delivering a public lecture on the subject. He hesitated very much to take up the suggestion, from the fear that the task would prove too formidable for his time and resources ; but on the other hand, from a sympathetic feeling towards all that concerned the past and the present of the parish, he resolved to proceed and do his best to collect and record in a permanent form such details as could be gathered from the various sources of information. Had the idea of collecting materials for such a work been entertained forty or forty-five years ago, the author could have given with greater fulness and accuracy a record of local history and traditionary incidents now forgotten, by committing to writing the recollections of old people living, many of whose traditionary tales have now escaped his memory. — While the indulgence of the reader is craved for errors detected or mistakes discovered, neither pains nor labour have been spared to make the History as full and correct as possible. -

Iv. Archbological Gleanings from Dark-Age Records. by Angus Graham
64 PROCEEDINGS OF THE SOCIETY, 1950-51. IV. ARCHBOLOGICAL GLEANINGS FROM DARK-AGE RECORDS. BY ANGUS GRAHAM, M.A., F.S.A., F.S.A.SCOT. CONTENTS. PdGll PmE I. INTRODUCTORY . 64 V. CHURCHES . 82 II. FORTS . 65 VI. TOWNS AND VILLAGES . 84 1. Nomenclature . 65 VII. MISCELLANEOUS . 87 2. Surviving Examples 68 1. Burials, Cairns, etc. 87 3. Materials, Dimensions, 2. Crosses and Sculpture 87 et& . 69 3. Standing-stones . 88 4. Ownership . 72 4. Crannogs . 88 5. Tactical Significance . 73 5. Earth-houses . 89 III. HOUSES . 74 6. Roads . 89 IV; MONASTERIES . 79 VIII. CONCLUDING NOTE. 90 I. INTRODUCTORY. The Dark-Age texts that bear on the history of Scotland have passed through fine nets of criticism and annotation, but their exponents’ main interests have often been in history, ecclesiology and language, with the result that archaeological questions have received less attention than they properly deserve. Again, in respect of the Irish material, which may on occasion be importa& for the purposes of comparison, it has happened that some scholars have tended to slur distinctions between earlier and later periods, and consequently the “ancient ” Ireland of which they present a picture is one of which certain features persisted right down to. the seven- teenth century. It is obvious that such matter can only be used with great caution if the intention is to illustrate, say, the Dalriada of a thousand years earlier. !L’he notes that follow have accordingly been prepared with the ARCHZEOLOGICAL GLEANINGS FROM DARK-AGE RECORDS. 65 idea of isolating, as far as possible, matter which is truly relevant to Dark- Age archaeology in Scotland ; and to achieve this end a range of early sources was selected for study which seemed likely to be relatively free from mediaeval infiltrations. -

Reassessing the Long Chronology of the Penannular Brooch in Britain: Exploring Changing Styles, Use and Meaning Across a Millennium
Reassessing the long chronology of the penannular brooch in Britain: exploring changing styles, use and meaning across a millennium Thesis submitted for the degree of Doctor of Philosophy At The University of Leicester By Anna Louise Booth School of Archaeology and Ancient History University of Leicester 2014 1 | P a g e Abstract Title: Reassessing the long chronology of the penannular brooch in Britain: exploring changing styles, use and meaning across a millennium Author: Anna Louise Booth Penannular brooches are a simple form of dress fastener used in Britain from the late Iron Age, through to the Roman and Early Medieval periods. This thesis represents the first full study of their British development for fifty years. The catalogue of penannulars originally compiled by Elizabeth Fowler in the late 1950s has been more than doubled, allowing a thorough re-analysis of chronological variation and continuity in stylistic development, distribution, use and deposition. This has been carried out via broad analysis of the penannular database and two regional case studies looking at South- West England and Yorkshire and North Lincolnshire, the two areas where penannulars were concentrated throughout their chronology. Many previous studies have focused only on the later penannular types, leading to an unbalanced approach dominated by the preoccupations of early medieval archaeology. This has created the perception that penannulars had a simple evolutionary development that contributed to the straightforward survival of a ‘Celtic’ culture in some regions during the Roman period and beyond. To counterbalance this, analysis here has particularly focused on the earlier end of the penannular chronology. As a result an alternative picture is presented, of a highly complex development influenced by Continental parallels, which stands in deep contrast to the simplistic sequences proposed in most previous studies.