Thesis Reference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bon Echo Provincial Park
BON ECHO PROVINCIAL PARK One Malaise trap was deployed at Bon Echo Provincial Park in 2014 (44.89405, -77.19691 278m ASL; Figure 1). This trap collected arthropods for twenty weeks from May 7 – September 24, 2014. All 10 Malaise trap samples were processed; every other sample was analyzed using the individual specimen protocol while the second half was analyzed via bulk analysis. A total of 2559 BINs were obtained. Over half the BINs captured were flies (Diptera), followed by bees, ants and wasps (Hymenoptera), moths and butterflies (Lepidoptera), and beetles (Coleoptera; Figure 2). In total, 547 arthropod species were named, representing 22.9% of the BINs from the site (Appendix 1). All BINs were assigned at least to Figure 1. Malaise trap deployed at Bon Echo family, and 57.2% were assigned to a genus (Appendix Provincial Park in 2014. 2). Specimens collected from Bon Echo represent 223 different families and 651 genera. Diptera Hymenoptera Lepidoptera Coleoptera Hemiptera Mesostigmata Trombidiformes Psocodea Sarcoptiformes Trichoptera Araneae Entomobryomorpha Symphypleona Thysanoptera Neuroptera Opiliones Mecoptera Orthoptera Plecoptera Julida Odonata Stylommatophora Figure 2. Taxonomy breakdown of BINs captured in the Malaise trap at Bon Echo. APPENDIX 1. TAXONOMY REPORT Class Order Family Genus Species Arachnida Araneae Clubionidae Clubiona Clubiona obesa Linyphiidae Ceraticelus Ceraticelus atriceps Neriene Neriene radiata Philodromidae Philodromus Salticidae Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha Tetragnatha shoshone -

Addenda and Amendments to a Checklist of the Lepidoptera of the British Isles on Account of Subsequently Published Data
Ent Rec 128(2)_Layout 1 22/03/2016 12:53 Page 98 94 Entomologist’s Rec. J. Var. 128 (2016) ADDENDA AND AMENDMENTS TO A CHECKLIST OF THE LEPIDOPTERA OF THE BRITISH ISLES ON ACCOUNT OF SUBSEQUENTLY PUBLISHED DATA 1 DAVID J. L. A GASSIZ , 2 S. D. B EAVAN & 1 R. J. H ECKFORD 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD 2 The Hayes, Zeal Monachorum, Devon EX17 6DF This update incorpotes information published before 25 March 2016 into A Checklist of the Lepidoptera of the British Isles, 2013. CENSUS The number of species now recorded from the British Isles stands at 2535 of which 57 are thought to be extinct and in addition there are 177 adventive species. CHANGE OF STATUS (no longer extinct) p. 17 16.013 remove X, Hall (2013) p. 25 35.006 remove X, Beavan & Heckford (2014) p. 40 45.024 remove X, Wilton (2014) p. 54 49.340 remove X, Manning (2015) ADDITIONAL SPECIES in main list 12.0047 Infurcitinea teriolella (Amsel, 1954) E S W I C 15.0321 Parornix atripalpella Wahlström, 1979 E S W I C 15.0861 Phyllonorycter apparella (Herrich-Schäffer, 1855) E S W I C 15.0862 Phyllonorycter pastorella (Zeller, 1846) E S W I C 27.0021 Oegoconia novimundi (Busck, 1915) E S W I C 35.0299 Helcystogramma triannulella (Herrich-Sch äffer, 1854) E S W I C 41.0041 Blastobasis maroccanella Amsel, 1952 E S W I C 48.0071 Choreutis nemorana (Hübner, 1799) E S W I C 49.0371 Clepsis dumicolana (Zeller, 1847) E S W I C 49.2001 TETRAMOERA Diakonoff, [1968] langmaidi Plant, 2014 E S W I C 62.0151 Delplanqueia inscriptella (Duponchel, 1836) E S W I C 72.0061 Hypena lividalis (Hübner, 1790) Chevron Snout E S W I C 70.2841 PUNGELARIA Rougemont, 1903 capreolaria ([Denis & Schiffermüller], 1775) Banded Pine Carpet E S W I C 72.0211 HYPHANTRIA Harris, 1841 cunea (Drury, 1773) Autumn Webworm E S W I C 73.0041 Thysanoplusia daubei (Boisduval, 1840) Boathouse Gem E S W I C 73.0301 Aedia funesta (Esper, 1786) Druid E S W I C Ent Rec 128(2)_Layout 1 22/03/2016 12:53 Page 99 Entomologist’s Rec. -

Butterflies and Moths of Union County, Oregon, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

Lepidoptera Recorded for Imperial County California Compiled by Jeffrey Caldwell [email protected] 1-925-949-8696 Note
Lepidoptera Recorded for Imperial County California Compiled by Jeffrey Caldwell [email protected] 1-925-949-8696 Note: BMNA = Butterflies and Moths of North America web site MPG = Moth Photographers Group web site Most are from the Essig Museum’s California Moth Specimens Database web site Arctiidae. Tiger and Lichen Moths. Apantesis proxima (Notarctia proxima). Mexican Tiger Moth. 8181 [BMNA] Ectypia clio (clio). Clio Tiger Moth. 8249 Estigmene acrea (acrea). Salt Marsh Moth. 8131 Euchaetes zella. 8232 Autostichidae (Deoclonidae). Oegoconia novimundi. Four-spotted Yellowneck Moth. 1134 (Oegoconia quadripuncta mis-applied) Bucculatricidae. Ribbed Cocoon-maker Moths. Bucculatrix enceliae. Brittlebrush Moth. 0546 Cossidae. Goat Moths, Carpenterworm Moths, and Leopard Moths. Comadia henrici. 2679 Givira mucida. 2660 Hypopta palmata. 2656 Prionoxystus robiniae (mixtus). Carpenterworm or Locust Borer. 2693 Depressariidae. Pseudethmia protuberans. 1008 [MPG] Ethmiidae. Now assigned to Depressariidae. Ethmiinae. Ethmia timberlakei. 0984 Pseudethmia protuberans. 1008 Gelechiidae. Twirler Moths. Aristotelia adceanotha. 1726 [Sighting 1019513 BMNA] Chionodes abdominella. 2054 Chionodes dentella. 2071 Chionodes fructuaria. 2078 Chionodes kincaidella. 2086 (reared from Atriplex acanthocarpa in Texas) Chionodes oecus. 2086.2 Chionodes sistrella. 2116 Chionodes xanthophilella. 2125 Faculta inaequalis. Palo Verde Webworm. 2206 Friseria cockerelli. Mesquite Webworm. 1916 Gelechia desiliens. 1938 Isophrictis sabulella. 1701 Keiferia lycopersicella. Tomato Pinworm. 2047 Pectinophora gossypiella. Pink Bollworm. 2261 Prolita puertella. 1895 Prolita veledae. 1903 Geometridae. Inchworm Moths, Loopers, Geometers, or Measuring Worms. Archirhoe neomexicana. 7295 Chesiadodes coniferaria. 6535 Chlorochlamys appellaria. 7073 Cyclophora nanaria. Dwarf Tawny Wave. W 7140 Dichorda illustraria. 7055 Dichordophora phoenix. Phoenix Emerald. 7057 Digrammia colorata. Creosote Moth. 6381 Digrammia irrorata (rubricata). 6395 Digrammia pictipennata. 6372 Digrammia puertata. -

Mise En Page 1
REVUE SUISSE DE ZOOLOGIE Tome 113 — Fascicule 2 Pages Ding YANG, Bernhard MERZ & Patrick GROOTAERT. Descriptions of three new Platypalpus Macquart from Guangdong, China (Diptera, Hybotidae, Tachydromiinae). 229-238 Wolfgang SCHAWALLER. New species and records of the genus Basanus Lacordaire (Insecta: Coleoptera: Tenebrionidae) . 239-246 Wilson R. LOURENÇO & Steven M. GOODMAN. Further considerations regarding the status of Grosphus madagascariensis (Gervais) and Grosphus hirtus Kraepelin, and description of a new species (Scorpiones, Buthidae) . 247-261 Adrian SMOLIS & Louis DEHARVENG. Vitronura mascula, a new species of Neanurinae (Collembola: Neanuridae) from northern Vietnam, with a key to the species of the genus . 263-268 Daniel BURCKHARDT & Pavel LAUTERER. The Palaearctic triozids associated with Rubiaceae (Hemiptera, Psylloidea): a taxonomic re-evaluation of the Trioza galii Foerster complex . 269-286 Koen MAES. A new species of Diathrausta Lederer, 1863 from Africa (Lepidoptera, Pyraloidea, Crambidae, Spilomelinae). 287-290 Dariusz SKARZYNSKI. Redescription of Ceratophysella lawrencei (Gisin, 1963) (Collembola: Hypogastruridae) . 291-296 Dariusz SKARZYNSKI & Adrian SMOLIS. Description of Ceratophysella robustiseta sp. n. from greenhouses in England, with notes on synonymy of C. postanten- nalis Yosii, 1966 and taxonomic status of C. morula Deharveng & Bourgeois, 1991 (Collembola: Hypogastruridae) . 297-303 Juan Marcos MIRANDE, Gastón AGUILERA & Maria de las Mercedes AZPELICUETA. Nomenclatural note on the genus Nans (Ostariophysi, Characidae) . 305 Bernard LANDRY, David ADAMSKI, Patrick SCHMITZ, Christine E. PARENT & Lazaro ROQUE-ALBELO. Taygete sphecophila (Meyrick) (Lepidoptera; Autostichidae): redescription of the adult, description of the larva and pupa, and impact on Polistes wasps (Hymenoptera; Vespidae) nests in the Galapagos Islands . 307-323 Peter SCHUCHERT. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata Part 1 . -
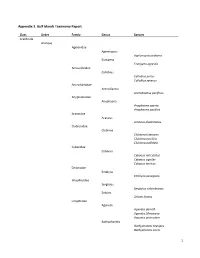
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey
1 Checklist of Texas Lepidoptera Knudson & Bordelon, Jan 2018 Texas Lepidoptera Survey ERIOCRANIOIDEA TISCHERIOIDEA ERIOCRANIIDAE TISCHERIIDAE Dyseriocrania griseocapitella (Wlsm.) Eriocraniella mediabulla Davis Coptotriche citripennella (Clem.) Eriocraniella platyptera Davis Coptotriche concolor (Zell.) Coptotriche purinosella (Cham.) Coptotriche clemensella (Cham). Coptotriche sulphurea (F&B) NEPTICULOIDEA Coptotriche zelleriella (Clem.) Tischeria quercitella Clem. NEPTICULIDAE Coptotriche malifoliella (Clem.) Coptotriche crataegifoliae (Braun) Ectoedemia platanella (Clem.) Coptotriche roseticola (F&B) Ectoedemia rubifoliella (Clem.) Coptotriche aenea (F&B) Ectoedemia ulmella (Braun) Asterotriche solidaginifoliella (Clem.) Ectoedemia obrutella (Zell.) Asterotriche heliopsisella (Cham.) Ectoedemia grandisella (Cham.) Asterotriche ambrosiaeella (Cham.) Nepticula macrocarpae Free. Asterotriche helianthi (F&B) Stigmella scintillans (Braun) Asterotriche heteroterae (F&B) Stigmella rhoifoliella (Braun) Asterotriche longeciliata (F&B) Stigmella rhamnicola (Braun) Asterotriche omissa (Braun) Stigmella villosella (Clem.) Asterotriche pulvella (Cham.) Stigmella apicialbella (Cham.) Stigmella populetorum (F&B) Stigmella saginella (Clem.) INCURVARIOIDEA Stigmella nigriverticella (Cham.) Stigmella flavipedella (Braun) PRODOXIDAE Stigmella ostryaefoliella (Clem.) Stigmella myricafoliella (Busck) Tegeticula yuccasella (Riley) Stigmella juglandifoliella (Clem.) Tegeticula baccatella Pellmyr Stigmella unifasciella (Cham.) Tegeticula carnerosanella Pellmyr -

Pests of Cultivated Plants in Finland
ANNALES AGRICULTURAE FE,NNIAE Maatalouden tutkimuskeskuksen aikakauskirja Vol. 1 1962 Supplementum 1 (English edition) Seria ANIMALIA NOCENTIA N. 5 — Sarja TUHOELÄIMET n:o 5 Reprinted from Acta Entomologica Fennica 19 PESTS OF CULTIVATED PLANTS IN FINLAND NIILO A.VAPPULA Agricultural Research Centre, Department of Pest Investigation, Tikkurila, Finland HELSINKI 1965 ANNALES AGRICULTURAE FENNIAE Maatalouden tutkimuskeskuksen aikakauskirja journal of the Agricultural Researeh Centre TOIMITUSNEUVOSTO JA TOIMITUS EDITORIAL BOARD AND STAFF E. A. jamalainen V. Kanervo K. Multamäki 0. Ring M. Salonen M. Sillanpää J. Säkö V.Vainikainen 0. Valle V. U. Mustonen Päätoimittaja Toimitussihteeri Editor-in-chief Managing editor Ilmestyy 4-6 numeroa vuodessa; ajoittain lisänidoksia Issued as 4-6 numbers yearly and occasional supplements SARJAT— SERIES Agrogeologia, -chimica et -physica — Maaperä, lannoitus ja muokkaus Agricultura — Kasvinviljely Horticultura — Puutarhanviljely Phytopathologia — Kasvitaudit Animalia domestica — Kotieläimet Animalia nocentia — Tuhoeläimet JAKELU JA VAIHTOTI LAUKS ET DISTRIBUTION AND EXCHANGE Maatalouden tutkimuskeskus, kirjasto, Tikkurila Agricultural Research Centre, Library, Tikkurila, Finland ANNALES AGRICULTURAE FENNIAE Maatalouden tutkimuskeskuksen aikakauskirja 1962 Supplementum 1 (English edition) Vol. 1 Seria ANIMALIA NOCENTIA N. 5 — Sarja TUHOELÄIMET n:o 5 Reprinted from Acta Entomologica Fennica 19 PESTS OF CULTIVATED PLANTS IN FINLAND NIILO A. VAPPULA Agricultural Research Centre, Department of Pest Investigation, -

FOLIA ENTOMOLOGICA HUNGARICA ROVARTANI KÖZLEMÉNYEK Volume 70 2009 Pp
FOLIA ENTOMOLOGICA HUNGARICA ROVARTANI KÖZLEMÉNYEK Volume 70 2009 pp. 139−146 New data to the Microlepidoptera fauna of Hungary, part XII CS. SZABÓKY1, Z. TOKÁR2, J. LIŠKA3 & G. PASTORÁLIS4 1 H-1034 Budapest, Bécsi út 88, Hungary. E-mail: [email protected] 2 P.J. Šafárika 11, SK-92701 Ša¾a, Slovakia. E-mail: [email protected] 3 Strnady 138, CZ-15604 Praha 5 - Zbraslav, Czechia. E-mail: [email protected] 4 Košická 22/39, SK-94501 Komárno, Slovakia. E-mail: [email protected] – Seventeen species are new for the Hungarian Microlepidoptera fauna: Lypusa tokari ELSNER,LIŠKA et PETRÙ, 2008, Bucculatrix argentisignella HERRICH-SCHÄFFER, 1855, Bucculatrix herbalbella CHRÉTIEN, 1898, Bucculatrix pannonica DESCHKA, 1982, Oc- nerostoma piniariella ZELLER, 1847, Agonopterix conterminella (ZELLER, 1839), Coleophora impalella TOLL, 1961, Coleophora hartigi TOLL, 1944, Coleophora lessinica BALDIZZONE, 1980, Coleophora paucinotella TOLL, 1961, Hypatopa segnella (ZELLER, 1873), Oegoconia novimundi (BUSCK, 1915), Apatema apolausticum GOZMÁNY, 1996, Anarsia eleagnella KUZNETZOV, 1957, Acleris abietana (HÜBNER, 1822), Dichrorampha sedatana BUSCK, 1906, Gypsonoma obraztsovi AMSEL, 1959. The former Hungarian records of four species, Coleophora violacea (STRÖM, 1783), Coleophora serratulella HERRICH-SCHÄFFER, 1854, Athrips amoenella (FREY, 1882), Scrobipalpa arenbergeri POVOLNÝ, 1973 are confirmed. The newly recorded species are added to a revised on-line checklist of Microlepidoptera of Hungary. Caryocolum trauniella (ZELLER, 1868) is deleted from the list as its record is based on an unfortunate misidentification. – Microlepidoptera, new records, confirmed records, Hungary, checklist. INTRODUCTION This report is the twelfth contribution to the Microlepidoptera fauna of Hungary. Information is given about new and confirmed records of Folia ent. hung. 70, 2009 140 Cs. -

02 October 2015 Radebeul-Germany
©Societas Europaea Lepidopterologica; download unter http://www.soceurlep.eu/ und www.zobodat.at XIXth European Congress Welcome .............................................................................................................................................................. 3 of Lepidopterology Programme ....................................................................................................................................................... 5 27 September – 02 October 2015 Monday, 28 September 2015 ........................................................................................................ 5 Radebeul · Germany Tuesday, 29 September 2015 ....................................................................................................... 7 Wednesday, 30 September 2015 ................................................................................................ 9 Thursday, 1 October 2015 ............................................................................................................ 10 Friday, 2 October 2015 ................................................................................................................... 14 Honouring Niels Peder Kristensen ............................................................................................... 15 Abstracts .......................................................................................................................................................... 16 Oral presentations .......................................................................................................................... -

303 Lepidoptera: Acrolepiidae Digitivalva Arnicella
Published December 15, 2010 Klapalekiana, 46: 231–235, 2010 ISSN 1210-6100 FAUNISTIC RECORDS FROM THE CZECH REPUBLIC – 303 Lepidoptera: Acrolepiidae Digitivalva arnicella (Heyden, 1863). Bohemia occ., Mariánská (5643), 800 m a.s.l., 15.–20.vii.1991, 2 ♂♂ (1 spec. genitalia examined), J. Skyva lgt., det. et coll.; Bohemia mer.: Arnoštov (7149), 880 m a.s.l., 22.vii.2009, 1 ♂ captured in Pinus rotundata forest with Arnica montana; Přední Zvonková, Pod Borkovou Nature Reserve (7350), 725 m a.s.l., 12.vii.2010, 1 ♂ (genitalia examined), both J. Šumpich lgt., det. et coll. Widely distributed in Europe, it is absent from the Iberian and Balkan Peninsula, islands and eastern countries (Gaedike 2009). From Bohemia it is known only on the basis of old vouchers from Františkovy Lázně (Ster- neck & Zimmermann 1933) and Zákupy and Stráž nad Nežárkou (Gaedike 1980). The only record from Moravia (from Hodonín-Zbrod in 1982) was published by Janovský & Gottwald (1990). Confirmed occurrence in Bohemia after more than 80 years. Gracillariidae Parornix szocsi (Gozmány, 1952). Moravia mer.: Kobylí-Lácary (7067), 20.iv.2009, 1 ♂ (genitalia examined), J. Liška lgt., det. et coll.; National Park Podyjí, Havraníky, Fládnitz- ské vřesoviště Natural Monument (7161), 11.v.2009, 1 ♂ (genitalia examined), J. Šumpich lgt., det. et coll. Rather poorly known species, recorded in Spain, Sardinia, Austria, Czech Republic, Slovakia, Hungary, Romania, and Russia (Buszko 2009). In the Czech Republic hitherto known only from Karlštejn in central Bohemia (Novák & Liška 1997). New species for Moravia. Scythrididae Scythris kasyi Hannemann, 1962. Moravia mer.: Kobylí-Lácary (7067), 27.v.2009, 1 ♂, 8.vi.2009, 2 ♂♂, ��������������14.vii.2010, 3 ♀ ♀; Bořetice-Zázmoníky (7066), 14.vii.2010, 1 ♂, ��������J. -

Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves
insects Article Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves Chiara Ferracini 1,* , Cristina Pogolotti 1, Giada Lentini 1, Valerio Saitta 1, Enrico Busato 1, Franco Rama 2 and Alberto Alma 1 1 Department of Agricultural, Forest and Food Sciences (DISAFA), University of Torino, Largo Paolo Braccini 2, 10095 Grugliasco, Italy; [email protected] (C.P.); [email protected] (G.L.); [email protected] (V.S.); [email protected] (E.B.); [email protected] (A.A.) 2 Biological Products Unit, Isagro S.p.A., Via Fauser, 28, 28100 Novara, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0116708700 Received: 9 October 2020; Accepted: 15 November 2020; Published: 17 November 2020 Simple Summary: Investigations were performed in 2018–2019 in chestnut groves in northern Italy to monitor the seasonal flight activity of Pammene fasciana (L.), Cydia fagiglandana (Zeller), and C. splendana (Hübner) with pheromone-baited traps. Commercially available and experimental pheromone blends were tested. Newly formed chestnut husks and fruits were randomly collected to evaluate damage. Damage was correlated with trap catches. P. fasciana was present in all the sites, while Cydia species were recorded in three of six sites, with differences in abundance related to pheromone blends studied. Several morphologically similar non-target species occurred, highlighting the risk of overestimating catches. Fruit damage did not correlate with trap captures, suggesting that monitoring probably underestimates the true size of the moths’ populations. These data contribute to ascertaining the presence of tortrix moths in northern Italian chestnut groves, and are important for planning specific control measures.