22629 Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Captive Orcas
Captive Orcas ‘Dying to Entertain You’ The Full Story A report for Whale and Dolphin Conservation Society (WDCS) Chippenham, UK Produced by Vanessa Williams Contents Introduction Section 1 The showbiz orca Section 2 Life in the wild FINgerprinting techniques. Community living. Social behaviour. Intelligence. Communication. Orca studies in other parts of the world. Fact file. Latest news on northern/southern residents. Section 3 The world orca trade Capture sites and methods. Legislation. Holding areas [USA/Canada /Iceland/Japan]. Effects of capture upon remaining animals. Potential future capture sites. Transport from the wild. Transport from tank to tank. “Orca laundering”. Breeding loan. Special deals. Section 4 Life in the tank Standards and regulations for captive display [USA/Canada/UK/Japan]. Conditions in captivity: Pool size. Pool design and water quality. Feeding. Acoustics and ambient noise. Social composition and companionship. Solitary confinement. Health of captive orcas: Survival rates and longevity. Causes of death. Stress. Aggressive behaviour towards other orcas. Aggression towards trainers. Section 5 Marine park myths Education. Conservation. Captive breeding. Research. Section 6 The display industry makes a killing Marketing the image. Lobbying. Dubious bedfellows. Drive fisheries. Over-capturing. Section 7 The times they are a-changing The future of marine parks. Changing climate of public opinion. Ethics. Alternatives to display. Whale watching. Cetacean-free facilities. Future of current captives. Release programmes. Section 8 Conclusions and recommendations Appendix: Location of current captives, and details of wild-caught orcas References The information contained in this report is believed to be correct at the time of last publication: 30th April 2001. Some information is inevitably date-sensitive: please notify the author with any comments or updated information. -

Killer Whale (Aka Orca) Orcinus Orca
PGAV Zoo Design SDT Animal of the Month Killer Whale (aka Orca) Orcinus orca 1. Animal Type: Mammal 2. Conservation Status: Endangered (Southern resident population only—Others: Status unknown) a. Range/Habitat i. From the open sea to coasts, from equator to polar areas. ii. The most distributed of all cetaceans, it occurs in all oceans and seas. 3. Size a. Male: 10 tons and 19’ long b. Female: 7.5 tons and 16’ long c. At birth: 6 ½ feet to 8 feet 4. Social Structure a. Killer whales are a social species living in pods of 3-25 b. Killer whales use their calls to coordinate hunting behavior and to maintain contact with other members of their pod. Clicks are used by all toothed whales for echolocation, and they may also be used to communicate. i. With orcas, several resident pods may join to form a community. Each pod has a unique dialect, similar to other pods in its community. It is quite different from the dialects of other pods with which they are not in contact. ii. Studies of the orca show the subtlety and variety of whale language. They use discrete calls – distinct sounds separated into characteristic types that can be heard 5 miles away. Those in the northwest use between 7-17 calls each. They make different noises when playing or socializing. 5. Reproduction a. Gestation period is thought to be 12-16 months, with most calves born between October and March. b. Orcas have long childhoods, reaching maturity about 15 years old. c. A female can produce as many as 5 calves over a 25 year period. -

While Rome Burns…A Report Into Conditions in the Zoos of Ontario
While Rome Burns … A Report into Conditions in the Zoos of Ontario Prepared by: Samantha Lindley, BVSc, MRCVS September 1998 Published by: Zoocheck Canada Inc. 2646 St. Clair Avenue East Toronto, Ontario M4B 3M1 (Canada) Ph (416) 285-1744 Fax (416) 285-4670 Email: [email protected] Web Site: www.zoocheck.com World Society for the Protection of Animals (WSPA) Canada 90 Eglinton Avenue East, Ste. 960 Toronto, Ontario M4P 1Y3(Canada) Ph (416) 369-0044 Fax (416) 369-0147 Email: [email protected] Web Site: www.wspa.ca While Rome Burns…A Report into Conditions in the Zoos of Ontario Table of Contents Foreword …………………………………………………………………………. i About the Author ………………………………………………………………… iii While Rome Burns . Introduction ……………………..……..……………….. 1 Zoos in Ontario: A Discussion ………………………………………………….. 3 The “Ideal” Zoo? ………………………………………………………………… 3 Get-aways ……………………………………………………………………….. 3 Safety and Security ……………………………………………………………… 3 Education ………………………………………………………………………… 4 Behaviour ………………………………………………………………………… 5 Stereotypic Behaviour ……………………………………………………………. 5 Stereotypic Behaviour vs. Displacement Behaviour …………………………….. 6 The Conservation Argument …………………………………………………….. 7 The Welfare Issue ……………………………………………………………….. 8 Zoos in Ontario Bergeron’s Exotic Animal Sanctuary …………………………………………… 11 Greenview Aviaries, Park and Zoo ……………………………………………… 15 The Killman Zoo ………………………………………………………………… 19 Lazy Acre Farm …………………………………………………………………. 25 Marineland of Canada …………………………………………………………… 29 Northwoods Buffalo and Exotic Animal Ranch ………………………………… 35 Pineridge -

Stopping the Use, Sale and Trade of Whales and Dolphins in Canada How Protection Is Consistent with WTO Obligations
Stopping the use, sale and trade of whales and dolphins in Canada How protection is consistent with WTO obligations Prepared by Leesteffy Jenkins, Attorney at Law 219 West Main St., P.O. Box 634 Hillsborough, NH 03244-0634 Phone (603) 464-4395 For ZOOCHECK CANADA INC. 3266 Yonge Street, Suite 1417 Toronto, Ontario M4N 3P6 (416) 285-1744 (p) (416) 285-4670 (f ) [email protected] www.zoocheck.com This legal analysis was commissioned by Zoocheck Canada as part of a research initiative looking into how trade laws impact the trade and use of whales and dolphins in Canada, and to educate the public about the same. Page 1 April 25, 2003 QUESTION PRESENTED Whether a Canadian ban on the import and export of live cetaceans, wild-caught, captive born or those caught earlier in the wild and now considered captive, would violate Canada's obligations pursuant to the World Trade Organization (WTO) Agreements. CONCLUSION It is my understanding that there is currently no specific Canadian legislation banning the import/export of live cetaceans. Based on the facts* presented to me, however, it is my opinion that such legislation could be enacted consistent with WTO rules. This memorandum attempts to outline both the conditions under which Canadian regulation of trade in live cetaceans may be consistent with the WTO Agreements, as well as provide some guidance in the crafting of future legislation. PEER REVIEW & COMMENTS Chris Wold, Steve Shrybman, Esq Clinical Professor of Law and Director Sack, Goldblatt, Mitchell International Environmental Law Project 20 Dundas Street West, Ste 1130 Northwestern School of Law Toronto, Ontario M5G 2G8 Lewis and Clark College Tel.: (416) 979-2235 10015 SW Terwilliger Blvd Email: [email protected] Portland, Oregon, U.S.A. -

Recommendations for the Care and Maintenance of Marine Mammals © Canadian Council on Animal Care, 2014
Recommendations for the care and maintenance of marine mammals © Canadian Council on Animal Care, 2014 ISBN: 978-0-919087-57-6 Canadian Council on Animal Care 190 O’Connor St., Suite 800 Ottawa ON K2P 2R3 http://www.ccac.ca Acknowledgements The development of the Recommendations for the care and maintenance of marine mammals was com- missioned to the Canadian Council on Animal Care (CCAC) by the Department of Fisheries and Oceans Canada (DFO). To complete this task, the CCAC Standards Committee created the ad hoc subcommittee on marine mammals. We acknowledge and thank all the members of the subcommittee who worked together to develop, review, and guide this document through to publication: Dr. Pierre-Yves Daoust, University of Prince Edward Island Mr. John Ford, Fisheries and Oceans Canada (DFO) Mr. Henrik Kreiberg, Fisheries and Oceans Canada (DFO) Dr. Clément Lanthier, Calgary Zoo Dr. Kay Mehren, Veterinarian Emeritus, Toronto Zoo Ms. Tracy Stewart, Marineland of Canada Mr. Clint Wright, Vancouver Aquarium Dr. Gilly Griffin, Canadian Council on Animal Care We would also like to take this opportunity to recognize the dedication and vision of Dr. Jon Lien (Memo- rial University, deceased 2010), the Committee’s first Chair. Jon played a pivotal role in the creation of this document. It was the Lien Report: A review of live- capture and captivity of marine mammals in Canada that inspired DFO to request that CCAC develop recommendations for the care and maintenance of marine mammals. His vision became a collective goal - to improve the quality of life for all marine mammals held in captivity. -

A Summary of the Effects of Captivity on Orcas
A Summary of the Effects of Captivity on Orcas PEOPLE FOR THE ETHICAL TREATMENT OF ANIMALS Contents The Eff ects of Captivity on Tilikum and Orcas Generally at SeaWorld…………..................................…………......3 I. Orcas Are Extremely Intelligent Mammals Whose Brains Are Highly Developed in Areas Responsible for Complex Cognitive Functions, Including Self-Awareness, Social Cognition, Culture, and Language …………………………………………...............................................................................................…...4 II. Tilikum Is Deprived of Every Facet of His Culture and the Opportunity to Engage in Natural Behavior, Causing Extreme Stress and Suff ering….…………….….......................................................5 A. The Tanks at SeaWorld Provide Inadequate Space and Result in Stress……….…...........................5 B. SeaWorld’s Constant Manipulation of Tilikum’s Social Structure Results in Stress.................7 C. The Tanks at SeaWorld Create a Distressing Acoustic Environment…….………..….........................9 III. The Stressors of the Captive Environment at SeaWorld Result in Aggressiveness, Self- Injury, and Other Physical and Behavioral Abnormalities………………….……..............................................10 A. Aggression Between Orcas and Between Orcas and Humans……..……………..............................……10 B. Stereotypic Behavior………………….……………………………………….......................................................................….…..13 1. Painful Dental Problems Caused by Chewing Metal Gates and Concrete Tanks.....14 2. -

AWI Comments on Permit Application
October 29, 2012 BY ELECTRONIC MAIL Submitted via http://www.regulations.gov NOAA-NMFS-2012-0158 Michael Payne, Chief National Marine Fisheries Service/NOAA Conservation and Education Division Office of Protected Resources 1315 East-West Highway Silver Spring, MD 20910 Dear Mr. Payne: RE: 77 FR 52694, File No. 17324 Proposed Import of Beluga Whales from Russia I am writing on behalf of the Animal Welfare Institute (AWI) to strongly oppose the request by Georgia Aquarium Inc. (GAI) for a public display permit to authorize the import of 18 wild-captured beluga whales from the Russian Federation to the United States pursuant to the Marine Mammal Protection Act of 1972 (MMPA; 16 U.S.C. 1361 et seq.) and the regulations governing the taking, importing, and exporting of marine mammals (50 CFR 216). AWI supports the arguments against the import submitted by other NGOS including The Humane Society of the United States, and Whale and Dolphin Conservation and endorses the statement signed by 60 international organizations. This letter provides several reasons why AWI believes GAI’s request does not conform to the issuance conditions of 50 CFR 216.34. Specifically, we will focus on the fact that GAI cannot adequately demonstrate any of the following three points: · The proposed transport methods, which include a complicated transfer between containers and planes in Belgium, are humane and do not present unnecessary risks to the health and welfare of the belugas (50 CFR 216.34 (a) (1)); · Its qualifications, facilities, and resources are adequate for the proper care and maintenance of the live belugas being transported (50 CFR 216.34 (a) (6)); and · The removal of the belugas from the wild will not likely have a significant adverse impact on the species or stock (50 CFR 216.34 (a) (4)). -

Nature's Newsletter
D V E A NATURE’S NEWSLETTER O N L I N E E D I T I O N Volume 13, Issue 2 2W www.dveaglealliance.org W I L D L I F E A N D T H E E N V I R O N M E N T Nature’s Newsletter 1 www.dveaglealliance.org Photograph provided by The Elephant Project Tilikum performing during a show at SeaWorld Orlando. © Photo from Shutterstock via Whale Sanctuary Project THE WHALE SANCTUARY PROJECT T U R N I N G T H E T I D E F O R C A P T I V E W H A L E S by Lori Marino, Ph.D. President, The Whale Sanctuary Project In 2010, a horrified world heard the news that a about these whales, captive facilities would eventually 12,500-pound male orca named Tilikum had killed trainer “get it right.” And while there was some improvement in Dawn Brancheau during a show at SeaWorld Orlando. Like survivorship of orcas taken into captivity over the first couple everyone else, I was heartbroken for the loss of Dawn’s life of decades, that same metric has been at a standstill for the but also because I knew that for Tilikum to commit such an past few recent decades. The scientific evidence of poor extreme violent act he must have been driven to the limits welfare, short lifespans, and psychological and physical of his ability to cope with years of captivity. He snapped. ailments continues to pile up, all pointing to the same conclusion: that the nature of orcas and other cetaceans is Those of us who study the wellbeing of captive orcas and entirely incompatible with life in the tanks. -
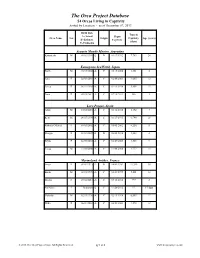
The Orca Project Database 54 Orcas Living in Captivity Sorted by Location - As of December 07, 2013
The Orca Project Database 54 Orcas Living in Captivity Sorted by Location - as of December 07, 2013 Birth Date Time in A=Actual Begin Orca Name Sex Origin Captivity Age (years) E=Estimate Captivity (days) U=Unknown Acuario Mundo Marino, Argentina Kshamenk M 01/01/1989 -E W 09/19/1992 7,749 24 Kamogawa SeaWorld, Japan Earth M 10/13/2008 -A C 10/13/2008 1,881 5 Lara F 02/08/2001 -A C 02/08/2001 4,685 12 Lovey F 01/11/1998 -A C 01/11/1998 5,809 15 Luna F 07/19/2012 -A C 07/19/2012 506 1 Loro Parque, Spain Adan M 10/12/2010 -A C 10/12/2010 1,152 3 Keto M 06/17/1995 -A C 06/17/1995 6,748 18 Kohana (Makea) F 05/03/2002 -A C 05/03/2002 4,236 11 Morgan F 01/01/2009 -E B 06/23/2010 1,263 4 Skyla F 02/09/2004 -A C 02/09/2004 3,589 9 Tekoa M 11/08/2000 -A C 11/08/2000 4,777 13 Marineland Antibes, France Freya F 01/01/1975 -E W 10/01/1982 11,390 38 Inouk M 02/23/1999 -A C 02/23/1999 5,401 14 Moana F 03/16/2011 -A C 03/16/2011 997 2 No Name ? Unknown -E C 11/20/2013 17 17 days Valentin M 02/13/1996 -A C 02/13/1996 6,507 17 Wikie F 06/01/2001 -A C 06/01/2001 4,572 12 © 2013 The Orca Project Corp. -

DOLPHIN EXPLOITATION and SUFFERING at SEAWORLD PARKS Heather D
© dreamstime.com/Paul Brewster © dreamstime.com/Paul DOLPHIN EXPLOITATION AND SUFFERING AT SEAWORLD PARKS Heather D. Rally, D.V.M. Supervising Veterinarian, PETA Foundation Toni Frohoff, Ph.D. Behavioral Biologist and Research Director, TerraMar Research JUNE 5, 2019 INTRODUCTION In 2016, SeaWorld announced that it would end its orca-breeding program after facing mounting criticism from scientists and the public over its poor treatment of captive orcas (Howard, 2016). Since this announcement, however, the company has continued to breed bottlenose and Pacific white-sided dolphins—the orca’s smaller cousins—and house them in small, concrete tanks, as well as exploiting them in archaic, circus-style performances and interactive programs in which they’re touched, grabbed, and stood on by trainers. In November 2018, a veterinarian observed the captive dolphins held at the SeaWorld parks in San Diego, San Antonio, and Orlando. Evidence obtained from those observations revealed that the well-being of captive dolphins at the parks is severely compromised by the conditions in which they’re forced to live. This includes an astonishing number of dolphins—140—confined to just seven tanks at four SeaWorld–owned theme parks in the Unites States.i This report provides a glimpse into the miserable lives of these exploited animals, who endure routine physical, psychological, and social stress and at times sustain painful injuries as a result. PEOPLE FOR THE ETHICAL TREATMENT OF ANIMALS | 1 GENERAL CONDITIONS Captive dolphins at SeaWorld are housed in severely crowded, extremely shallow, small enclosures that are spatially, acoustically, and visually unnatural to the species. Most have barren concrete walls with bright, reflective surfaces that prioritize maximum visibility and accessibility of dolphins for park visitors over consideration of the animals’ health and welfare. -

Consistent Motor Laterality in California Sea Lions
CONSISTENT MOTOR LATERALITY IN CALIFORNIA SEA LIONS Society for Marine Mammalogy Anne Janas, Paige McGrory, Dawn Oscvirk-Parliament and Michael Noonan Vancouver, British Columbia, Canisius College (Buffalo, New York) and Marineland of Canada (Niagara Falls, Ontario) November, 2001 Results Introduction High within measure reliability. In humans, such traits as left- and right-handedness are enduring characteristics of individuals, and Both measures were characterized by very high reliability across observations-both within and it is precisely their stability that accounts for their central place in theories pertaining to lateral across days (r = 0.7+, p < .0001). That is, individual Sea Lions had distinct preferences to favor cerebral dominance. Similar stability of left-right behaviors in marine mammals would likewise carry their left or right flipper leads and to prefer left or right circling in a way that was very consistent implications about cerebral asymmetry in these species. For this reason, we tested for consistent within animals over time. left/right motor biases in captive California Sea Lions. We assessed lateral bias in two settings—in foot-fall patterns when running and in direction of circling when swimming. Lack of population bias. Across the sample, there was no significant directional bias on either task—that is, on both measures there were approximately equal numbers of right- and left-biased animals. Neither were there reliable differences between the sexes on overall direction of bias. Low between measure correspondence. Across all subjects, the correlation between the two forms of locomotion (mean lateral bias across observations on each measure) was 0.24 (ns). Procedure Sex differences. -

Finding of No Significant Impact for Issuance of A
UNITED STATES DEPARTMENT OF COMMERCE NATIONAL MARINE FISHERIES SERVICE 1315 East-West Highway Silver Spring, Maryland 20910 FINDING OF NO SIGNIFICANT IMPACT FOR ISSUANCE OF A SCIENTIFIC RESEARCH PERMIT TO MYSTIC AQUARIUM FOR THE IMPORTATION AND TAKE OF CAPTIVE BELUGA WHALES (DELPHINAPTERUS LEUCAS) I. INTRODUCTION The National Marine Fisheries Service (NMFS) received an application (File No. 22629) from Mystic Aquarium requesting a permit to import five captive-born beluga whales (Delphinapterus leucas) from Marineland of Canada, Inc. (hereafter Marineland), located in Niagara Falls, Ontario, Canada, to Mystic Aquarium located in Mystic, Connecticut, for the purpose of conducting scientific research. NMFS is required to review applications and, if appropriate, may issue permits pursuant to the Marine Mammal Protection Act of 1972, as amended (MMPA) 1. In addition, the National Environmental Policy Act (NEPA), the Council on Environmental Quality Regulations (CEQ)2 and NOAA policy and procedures3 require all proposals for major federal actions be reviewed with respect to environmental consequences on the human environment. The purpose of this document is to present the evaluation that issuance of a scientific research permit to Mystic Aquarium will not significantly impact the quality of the human environment. This Finding of No Significant Impact (FONSI) and the Final Environmental Assessment (EA) titled “Issuance of Marine Mammal Protection Act Scientific Research Permit to Mystic Aquarium for the Importation and Take of Captive Beluga Whales (Delphinapterus leucas)” were prepared in accordance with CEQ Regulations and NOAA policy and procedures. II. BACKGROUND As described in the permit application and EA, Mystic Aquarium requested to import five captive-born beluga whales4 from Marineland to Mystic Aquarium for the purposes of conducting scientific research.