Variation in Salmonid Life Histories: Patterns and Perspectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Diversity of Fish (Pisces) in Freshwater
Hydrobiologia (2008) 595:545–567 DOI 10.1007/s10750-007-9034-0 FRESHWATER ANIMAL DIVERSITY ASSESSMENT Global diversity of fish (Pisces) in freshwater C. Le´veˆque Æ T. Oberdorff Æ D. Paugy Æ M. L. J. Stiassny Æ P. A. Tedesco Ó Springer Science+Business Media B.V. 2007 Abstract The precise number of extant fish spe- species live in lakes and rivers that cover only 1% cies remains to be determined. About 28,900 species of the earth’s surface, while the remaining 16,000 were listed in FishBase in 2005, but some experts species live in salt water covering a full 70%. While feel that the final total may be considerably higher. freshwater species belong to some 170 families (or Freshwater fishes comprise until now almost 13,000 207 if peripheral species are also considered), the species (and 2,513 genera) (including only fresh- bulk of species occur in a relatively few groups: water and strictly peripheral species), or about the Characiformes, Cypriniformes, Siluriformes, 15,000 if all species occurring from fresh to and Gymnotiformes, the Perciformes (noteably the brackishwaters are included. Noteworthy is the fact family Cichlidae), and the Cyprinodontiformes. that the estimated 13,000 strictly freshwater fish Biogeographically the distribution of strictly fresh- water species and genera are, respectively 4,035 species (705 genera) in the Neotropical region, 2,938 (390 genera) in the Afrotropical, 2,345 (440 Guest editors: E. V. Balian, C. Le´veˆque, H. Segers & K. Martens genera) in the Oriental, 1,844 (380 genera) in the Freshwater Animal Diversity Assessment Palaearctic, 1,411 (298 genera) in the Nearctic, and 261 (94 genera) in the Australian. -

Freshwater Ecosystems and Biodiversity
Network of Conservation Educators & Practitioners Freshwater Ecosystems and Biodiversity Author(s): Nathaniel P. Hitt, Lisa K. Bonneau, Kunjuraman V. Jayachandran, and Michael P. Marchetti Source: Lessons in Conservation, Vol. 5, pp. 5-16 Published by: Network of Conservation Educators and Practitioners, Center for Biodiversity and Conservation, American Museum of Natural History Stable URL: ncep.amnh.org/linc/ This article is featured in Lessons in Conservation, the official journal of the Network of Conservation Educators and Practitioners (NCEP). NCEP is a collaborative project of the American Museum of Natural History’s Center for Biodiversity and Conservation (CBC) and a number of institutions and individuals around the world. Lessons in Conservation is designed to introduce NCEP teaching and learning resources (or “modules”) to a broad audience. NCEP modules are designed for undergraduate and professional level education. These modules—and many more on a variety of conservation topics—are available for free download at our website, ncep.amnh.org. To learn more about NCEP, visit our website: ncep.amnh.org. All reproduction or distribution must provide full citation of the original work and provide a copyright notice as follows: “Copyright 2015, by the authors of the material and the Center for Biodiversity and Conservation of the American Museum of Natural History. All rights reserved.” Illustrations obtained from the American Museum of Natural History’s library: images.library.amnh.org/digital/ SYNTHESIS 5 Freshwater Ecosystems and Biodiversity Nathaniel P. Hitt1, Lisa K. Bonneau2, Kunjuraman V. Jayachandran3, and Michael P. Marchetti4 1U.S. Geological Survey, Leetown Science Center, USA, 2Metropolitan Community College-Blue River, USA, 3Kerala Agricultural University, India, 4School of Science, St. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Diversity and Longitudinal Distribution of Freshwater Fish in Klawing River, Central Java, Indonesia
BIODIVERSITAS ISSN: 1412-033X Volume 19, Number 1, January 2018 E-ISSN: 2085-4722 Pages: 85-92 DOI: 10.13057/biodiv/d190114 Diversity and longitudinal distribution of freshwater fish in Klawing River, Central Java, Indonesia SUHESTRI SURYANINGSIH♥, SRI SUKMANINGRUM, SORTA BASAR IDA SIMANJUNTAK, KUSBIYANTO Faculty of Biology, Universitas Jenderal Soedirman. Jl. Dr. Soeparno No. 63, Purwokerto-Banyumas 53122, Central Java, Indonesia. Tel.: +62-281- 638794, Fax.: +62-281-631700, ♥email: [email protected] Manuscript received: 10 July 2017. Revision accepted: 2 December 2017. Abstract. Suryaningsih S, Sukmaningrum S, Simanjuntak SBI, Kusbiyanto. 2018. Diversity and longitudinal distribution of freshwater fish in Klawing River, Central Java, Indonesia. Biodiversitas 19: 85-92. The aims of this study were to evaluate the diversity and longitudinal distribution of fish in Klawing River, Purbalingga (Central Java). The survey was performed using a clustered random- sampling technique. The river was divided into upstream, midstream and downstream regions. Species diversity was measured as the number of species, and the longitudinal distribution was assessed by determining the fish species present in each of the three regions. Eighteen fish species of eleven families were identified in the Klawing River: Cyprinidae, Bagridae, Mastacembelidae, Anabantidae, Cichlidae, Channidae, Eleotrididae, Beleontinidae, Osphronemidae, Poecilidae, and Siluridae. Cyprinidae exhibited the highest number of species (six), followed by Bagridae and Cichlidae (two species each). The other families were represented by one species each. A single cluster analysis showed that the upstream population had a similarity of 78% and 50% with the midstream and downstream populations, respectively. Species and family diversities were higher in the midstream populations than in the upstream and downstream populations. -

Вопросы Ихтиологии Том 59 № 4 2019 Июль–Август Основан В 1953 Г
Российская академия наук ВОПРОСЫ ИХТИОЛОГИИ Том 59 № 4 2019 Июль–Август Основан в 1953 г. Выходит 6 раз в год ISSN: 0042-8752 Журнал издается под руководством Отделения биологических наук РАН Редакционная коллегия: Главный редактор Д.С. Павлов А.М. Орлов (ответственный секретарь), С.А. Евсеенко (заместитель главного редактора), М.В. Мина (заместитель главного редактора), М.И. Шатуновский (заместитель главного редактора), О.Н. Маслова (научный редактор) Редакционный совет: П.-А. Амундсен (Норвегия), Д.А. Астахов, А.В. Балушкин, А.Е. Бобырев, Й. Вайценбок (Австрия), Ю.Ю. Дгебуадзе, А.В. Долгов, М. Докер (Канада), А.О. Касумян, Б. Коллетт (США), А.Н. Котляр, К.В. Кузищин, Е.В. Микодина, В.Н. Михеев, П. Моллер (США), А.Д. Мочек, Д.А. Павлов, Ю.С. Решетников, А.М. Токранов, В.П. Шунтов Зав. редакцией М.С. Чечёта E-mail: [email protected] Адрес редакции: 119071 Москва, Ленинский проспект, д. 33 Телефон: 495-958-12-60 Журнал “Вопросы ихтиологии” реферируется в Реферативном журнале ВИНИТИ, Russian Science Citation Index (Clarivate Analytics) Москва ООО «ИКЦ «АКАДЕМКНИГА» Оригинал-макет подготовлен ООО «ИКЦ «АКАДЕМКНИГА» © Российская академия наук, 2019 © Редколлегия журнала “Вопросы ихтиологии” (составитель), 2019 1 Подписано к печати 28.12.2018 г. Дата выхода в свет 15.03.2019 г. Формат 60 × 88 /8 Усл. печ. л. 15.5 Тираж 24 экз. Зак.2047 Бесплатно Учредитель: Российская академия наук Свидетельство о регистрации средства массовой информации ПИ № ФС77-66712 от 28 июля 2016 г., выдано Федеральной службой по надзору в сфере связи, информационных технологий и массовых коммуникаций (Роскомнадзор) Издатель: Российская академия наук, 119991 Москва, Ленинский пр., 14 Исполнитель по госконтракту № 4У-ЭА-197-18 ООО «ИКЦ «АКАДЕМКНИГА», 109028 Москва, Подкопаевский пер., 5, мезонин 1, к. -

Marine Fish Culture
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: © 1998 Kluwer. This manuscript is an author version with the final publication available and may be cited as: Tucker, J. W., Jr. (1998). Marine fish culture. Boston: Kluwer Academic Publishers. MARINE FISH CULTURE by John W. Tucker, Jr., Ph.D. Harbor Branch Oceanographic Institution and Florida Institute of Technology, Melbourne KLUWER ACADEMIC PUBLISHERS Boston I Dordrecht I London Distributors for North, Central and South America: Kluwer Academic Publishers I 01 Philip Drive Assinippi Park Norwell, Massachusetts 02061 USA Telephone (781) 871-6600 Fax (781) 871-6528 E-Mail <[email protected]> Distributors for all other countries: Kluwer Academic Publishers Group Distribution Centre Post Office Box 322 3300 AH Dordrecht, THE NETHERLANDS Telephone 31 78 6392 392 Fax 31 78 6546 474 E-Mail <[email protected]> '' Electronic Services <http://www.wkap.nl> Library of Congress Cataloging-in-Publication Data Tucker, John W., 1948- Marine fish culture I by John W. Tucker, Jr. p. em. Includes bibliographical references (p. ) and index. ISBN 0-412-07151-7 (alk. paper) 1. Marine fishes. 2. Fish-culture. I. Title. SH163.T835 1998 639.3'2--dc21 98-42062 CIP Copyright © 1998 by Kluwer Academic Publishers All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, photo copying, recording, or otherwise, without the prior written permission of the publisher, Kluwer Academic Publishers, I 0 I Philip Drive, Assinippi Park, Norwell, Massachusetts 02061 Printed on acid-free paper. -

Stenodus Leucichthys Nelma
Geomorphology and inconnu spawning site selection: an approach using GIS and remote sensing Item Type Thesis Authors Tanner, Theresa Lynn Download date 26/09/2021 20:09:43 Link to Item http://hdl.handle.net/11122/6998 (,!OMORPilOI OGY AND fNCONNl! SPAWNING SU P SIN I t : I H.D APPROACH USING GIS AND RE MOT I- SENSING By I hcrcsa I ynn banner EEC, OMMENDED: Dr. David Verbyla Dr. Mark S- Wipfli Dr. t .Joseph iVlargrab Advisory CAyiuniltec (hair Dr. William W. Smoker, Director, i asheries Division APPROVED; Dean, Schooiof Fisheries and OEgijn Sciences (Mm of the Graduate School I Ene GEOMORPHOLOGY AND INCONNU SPAWNING SITE SELECTION: AN APPROACH USING GIS AND REMOTE SENSING A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Theresa Lynn Tanner, A.S., B.S. Fairbanks, Alaska August 2008 iii ABSTRACT This study examined the spatial components of inconnu Stenodus leucichthys spawning habitat use in the Selawik River, Alaska. Little is known about inconnu critical habitat needs; however, current studies of inconnu spawning behavior suggest a high level of habitat selectivity. This level of selectivity implies that there are specific habitat characteristics that these fish require for spawning. The purpose of this study was to build a heuristic habitat model that can be used to better understand inconnu spawning site selection in remote Alaskan watersheds. Using readily available, low- or no-cost remote sensing data layers, geographical information systems (GIS) were used in conjunction with multivariate statistics in an attempt to clarify relationships between geomorphologic features and spawning site selection. -

Dominance and Predator Avoidance in Domesticated and Wild Masu Salmon Oncorhynchus Masou
Blackwell Science, LtdOxford, UK FISFisheries Science0919-92682003 Blackwell Science Asia Pty Ltd 691February 2003 591 Behavior of domesticated salmon T Yamamoto and UG Reinhardt 10.1046/j.0919-9268.2002.00591.x Original Article8894BEES SGML FISHERIES SCIENCE 2003; 69: 88–94 Dominance and predator avoidance in domesticated and wild masu salmon Oncorhynchus masou Toshiaki YAMAMOTO* AND Ulrich G REINHARDTa Laboratory of Conservation Biology, Field Science Center for Northern Biosphere, Hokkaido University, Sapporo, Hokkaido 060-0809, Japan ABSTRACT: Dominance, aggression and predator avoidance were compared among farmed, sea- ranched and wild juvenile masu salmon Oncorhynchus masou in laboratory experiments. Domesti- cated fish (farmed and sea-ranched), which had been exposed to artificial selection, were not dominant against wild fish in pairwise contests, nor did they show greater aggressiveness. Farmed fish did show greater feeding than wild fish. Under chemically simulated predation risk, farmed fish were more willing to leave cover and feed than wild fish, indicating reduced predator avoidance in the farmed fish. Our results indicate that selection for fast growth (domestication) in masu salmon favors fish that respond to food quickly and ignore predation risk. KEY WORDS: aggressive behavior, hatchery, masu salmon, predator avoidance. INTRODUCTION impacts the growth of individuals.9 In other salmo- nids, aggressiveness of domesticated juveniles has Numerous studies on salmonids have investigated been shown to increase.10 Therefore, introduced the differences in morphology, genetics and masu juveniles may influence wild populations behavior between domesticated and wild fish of directly through aggressive contests for territories. the same species.1–4 It has become clear that the As masu salmon show strong local adaptations,11,12 introduction of cultured fishes into rivers may there is concern that the introduction of domesti- have negative effects on wild fish populations. -

Comment on G Marty Dcoument
Critique of the Document “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions” Presented to the Provincial Government of British Columbia by Gary Marty, D.V.M., Ph.D., Diplomate, A.C.V.P. of the British Columbia Ministry of Agriculture, Animal Health Centre, Abbotsford. Authors of this critique: Lawrence M. Dill1, Martin Krkosek2, Brendan Connors3, Stephanie J. Peacock4, Andrew W. Bateman5, Richard Routledge6, Mark A. Lewis7, and John Reynolds8 1 Professor Emeritus, Department of Biological Sciences, Simon Fraser University 2 Assistant Professor, Department of Ecology and Evolutionary Biology, University of Toronto 3 Senior Systems Ecologist, ESSA Technologies, and Adjunct Professor, Department of Biological Sciences, Simon Fraser University 4 PhD Candidate, Department of Biological Sciences, University of Alberta 5 Postdoctoral Fellow, Department of Biological Sciences, University of Alberta and Department of Ecology and Evolutionary Biology, University of Toronto 6 Professor, Department of Statistics and Actuarial Science, Simon Fraser University 7 Professor and Senior Canada Research Chair, Departments of Biological Sciences and Mathematical and Statistical Sciences, University of Alberta 8 Professor and Tom Buell BC Leadership Chair in Aquatic Conservation, Department of Biological Sciences, Simon Fraser University Background The document, “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions,” dated March 16, 2015, was presented to Ministers Thompson and Letnik of the Government of British Columbia (BC) with the intention of providing scientific information upon which to base management and policy decisions regarding wild and farmed salmon in British Columbia. Collectively, we are a group of scientists, mostly academic, whose research expertise includes salmon and infectious diseases (here we refer to infectious diseases in the broadest sense as those that may arise from parasitic, viral or bacterial pathogens). -
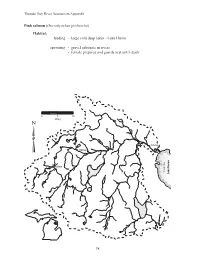
Lake Huron Spawning
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

Geomorphology and Inconnu Spawning Site Selection: An
GEOMORPHOLOGY AND INCONNU SPAWNING SITE SELECTION: AN APPROACH USING GIS AND REMOTE SENSING A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Theresa Lynn Tanner, A.S., B.S. Fairbanks, Alaska August 2008 iii ABSTRACT This study examined the spatial components of inconnu Stenodus leucichthys spawning habitat use in the Selawik River, Alaska. Little is known about inconnu critical habitat needs; however, current studies of inconnu spawning behavior suggest a high level of habitat selectivity. This level of selectivity implies that there are specific habitat characteristics that these fish require for spawning. The purpose of this study was to build a heuristic habitat model that can be used to better understand inconnu spawning site selection in remote Alaskan watersheds. Using readily available, low- or no-cost remote sensing data layers, geographical information systems (GIS) were used in conjunction with multivariate statistics in an attempt to clarify relationships between geomorphologic features and spawning site selection. Spatial resolution of the remotely sensed data available in this study did not provide sufficient spatial detail to generate statistical correlations between spawning habitat selection and landscape characterizations. However, spawning occurred in areas of transition from high to low gradients, and in reaches typified as having very low slopes with very high sinuosity. Additionally, exploratory use of Radarsat