Case Report Primary Rete Testis Adenocarcinoma: a Report of 2 Cases and Literature Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

PROSTATE and TESTIS PATHOLOGY “A Coin Has Two Sides”, the Duality of Male Pathology
7/12/2017 PROSTATE AND TESTIS PATHOLOGY “A Coin Has Two Sides”, The Duality Of Male Pathology • Jaime Furman, M.D. • Pathology Reference Laboratory San Antonio. • Clinical Assistant Professor Departments of Pathology and Urology, UT Health San Antonio. Source: http://themoderngoddess.com/blog/spring‐equinox‐balance‐in‐motion/ I am Colombian and speak English with a Spanish accent! o Shannon Alporta o Lindsey Sinn o Joe Nosito o Megan Bindseil o Kandace Michael o Savannah McDonald Source: http://www.taringa.net/posts/humor/7967911/Sindrome‐de‐la‐ Tiza.html 1 7/12/2017 The Prostate Axial view Base Apex Middle Apex Sagittal view Reference: Vikas Kundra, M.D., Ph.D. , Surena F. Matin, M.D. , Deborah A. Kuban, M.Dhttps://clinicalgate.com/prostate‐cancer‐4/ Ultrasound‐guided biopsy following a specified grid pattern of biopsies remains the standard of care. This approach misses 21% to 28% of prostate cancers. JAMA. 2017;317(24):2532‐2542. http://www.nature.com/nrurol/journal/v10/n12/abs/nrurol.2013.195.html Prostate Pathology Inflammation / granulomas Categories Adenosis, radiation, atrophy seminal vesicle Biopsy Benign TURP HGPIN Unsuspected carcinoma is seen in 12% of Atypical IHC TURP cases. glands Prostatectomy Subtype, Gleason, Malignant fat invasion, vascular invasion Other malignancies: sarcomas, lymphomas Benign Prostate Remember Malignant Glands Lack Basal Glands Cells Basal cells Secretory cells Stroma 2 7/12/2017 Benign Prostatic Lesions Atrophy Corpora amylacea (secretions) Seminal Vesicle Acute inflammation GMS Basal cell hyperplasia Basal cell hyperplasia Granulomas (BPH) (BPH) coccidiomycosis Mimics of Prostate Carcinoma Atrophy. Benign Carcinoma with atrophic features Prostate Carcinoma 1. Prostate cancer is the most common, noncutaneous cancer in men in the United States. -

Fluid on the Germinal Epithelium in the Rat Lynn M
Effects of obstruction of the flow of seminiferous tubule fluid on the germinal epithelium in the rat Lynn M. C. Pilsworth, B. T. Hinton and B. P. Setchell Department ofPhysiology, A.R.C. Institute ofAnimal Physiology, Babraham, Cambridge CB2 4AT, UK. Summary. Blocking the lumen of a single seminiferous tubule by introducing a plug of non-toxic latex produced a lesion in that tubule, but not in immediately adjacent tubules. The lesion extended for up to 50 mm from the end of the latex. Nearest to the block the tubule was completely aspermatogenic; further along the tubule, the lesion was less severe, involving disorganization and reduction in germ cell numbers, with the cells showing vacuolation, pycnosis and karyolysis. Binucleate and giant cells were common, and cells were often exfoliated into the lumen. The lesion tended to increase in length with longer times after introduction of the plug, but there appeared to be no preferential involvement of the shorter segment of the tubule between the block and the rete. The transition from damaged to healthy tubule was abrupt. Introduction The blood-testis barrier controls the composition of the seminiferous tubule fluid, which is thought to be important for the nurture of germ cells. Its composition probably reflects the conditions within the adluminal compartment of the germinal epithelium in which meiosis is completed (Setchell, 1970, 1978, 1980; Setchell & Waites, 1975). In addition, a possible involvement of fluid in the entrainment of the spermatogenic wave has been suggested (Perey, Clermont & Leblond, 1961) and flow of fluid may be important in the regulation of spermatogenesis. -

Universidad Católica De Santa Maria
UNIVERSIDAD CATÓLICA DE SANTA MARIA FACULTAD DE CIENCIAS E INGENIERIAS BIOLOGICAS Y QUIMICAS PROGRAMA PROFESIONAL DE MEDICINA VETERINARIA Y ZOOTECNIA “Identificación ecográfica de quistes en rete testis de alpacas en edad reproductiva en la Asociación de Criadores de Alpacas Sector Phacco, Distrito de Pitumarca, Provincia de Canchis, Cusco 2013” “Sonographic identification of cysts in rete testis of alpacas reproductive age in Alpacas Breeders Association Sector Phacco, Pitumarca District, Canchis Province, Cusco 2013” Tesis presentada por la Bachiller: FERNANDA CAROLINA PRADO CAYO Para optar el Título Profesional de: MEDICO VETERINARIO Y ZOOTECNISTA AREQUIPA- PERÚ 2013 1 DEDICATORIA Dedico mi tesis a Dios, mis padres y a mi hermano que me acompañaron en todo momento, nunca me sentí sola porque los llevo en el corazón, gracias porque a pesar de la distancia siempre están aquí conmigo, porque se preocuparon tanto para que sea una profesional, gracias por su apoyo. 2 AGRADECIMIENTOS A Dios por ser mi guía cada día en mi vida diaria. A la Universidad Católica de Santa María por ser mi Alma Mater y el lugar que sentí como mi segundo hogar. Al Programa Profesional de Medicina Veterinaria y Zootecnia porque me brindo la confianza de realizar mi carrera profesional y desempeñarse así en la sociedad. A los Docentes, por compartir conmigo sus enseñanzas y su dedicación para lograr culminar mi carrera profesional. A mi Asesor Mg. Fernando Fernández Fernández por su apoyo incondicional durante el desarrollo de mi proyecto de investigación. A mis Jurados: Mg. Gary Villanueva Gandarillas, Mg. Guillermo Vásquez Rodríguez, Mg. Jorge Zegarra Paredes; por su dedicación, comprensión y enseñanzas que me aportaron durante este tiempo. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Male Reproductive Organs Testes (Paired Gonads)
Male Reproductive Organs Testes (paired Gonads) Penis Series of passageways . Epididymis . Ductus Deferens . Urethra Accessory Glands . Seminal vesicle . Prostate Functions • Paired Gonads (Testes) – Produce Spermatozoa (male germ cells) & Androgens (male sex hormones) •Penis– Copulatory organ • Series of passageways & ducts – To store the spermatozoa , ready for delivery to male copulatory organ • Male accessory glands – provide fluid vehicle for carrying spermatozoa Coverings Tunica Vaginalis Tunica Albuginea Tunica Vasculosa Outermost Layer . Tunica Albuginea (Dense connective tissue fibrous Memb.) – Consist of closely packed collagen Fibres with a few Elastic Fibres . form septa ,Project from Mediastinum Testis . Divide incompletely into pyramidal lobules with apex towards Mediatinum . Each Testis Approx-200 lobule . Each lobule has Approx1-4 seminiferous Tubules . Form loop to end in Straight tubule (20-30) • Straight tubules end up unite to form network (Rete testis) which gives off 15-20 efferent ductules • Space between tubules filled up by Loose connective tissue (collagen fibres & fibroblasts,macrophases , mast cells), blood vessels, Lymphatics & Interstitial cells of Leydig Seminiferous Tubules • Fill most of interior of Each Testes • Two types of cells • Germ cells (represent different stages of spermatogenesis) Spermatogonia (Type A & type B) Primary spermatocyte Secondary spermatocyte Spermatids Spermatozoa • Sustantacular cells (Sertoli) Mitosis Spermatogonium 44+X 44+X Type A +Y +Y Spermatogonium 44+X+ Y Type B Enlarge/Mitosis -
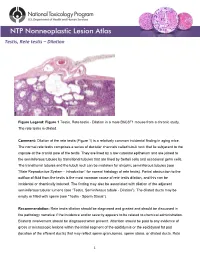
Testis, Rete Testis – Dilation
Testis, Rete testis – Dilation Figure Legend: Figure 1 Testis, Rete testis - Dilation in a male B6C3F1 mouse from a chronic study. The rete testis is dilated. Comment: Dilation of the rete testis (Figure 1) is a relatively common incidental finding in aging mice. The normal rete testis comprises a series of ductular channels called tubuli recti that lie subjacent to the capsule at the cranial pole of the testis. They are lined by a low cuboidal epithelium and are joined to the seminiferous tubules by transitional tubules that are lined by Sertoli cells and occasional germ cells. The transitional tubules and the tubuli recti can be mistaken for atrophic seminiferous tubules (see “Male Reproductive System - Introduction” for normal histology of rete testis). Partial obstruction to the outflow of fluid from the testis is the most common cause of rete testis dilation, and this can be incidental or chemically induced. The finding may also be associated with dilation of the adjacent seminiferous tubular lumens (see “Testis, Seminiferous tubule - Dilation”). The dilated ducts may be empty or filled with sperm (see “Testis - Sperm Stasis”). Recommendation: Rete testis dilation should be diagnosed and graded and should be discussed in the pathology narrative if the incidence and/or severity appears to be related to chemical administration. Bilateral involvement should be diagnosed when present. Attention should be paid to any evidence of gross or microscopic lesions within the initial segment of the epididymis or the epididymal fat pad (location of the efferent ducts) that may reflect sperm granulomas, sperm stasis, or dilated ducts. Rete 1 Testis, Rete testis – Dilation testis dilation may be associated with seminiferous tubular dilation and/or germinal epithelial atrophy, since these may be a consequence of obstruction of fluid outflow. -

The “Road Map”
PRACTICAL ROADMAP MALE REPRODUCTIVE SYSTEM DR N GRAVETT THE TESTIS • Slide 7 Stain: Iron Haematoxylin NOTE: Iron haematoxylin, a blue-black stain demonstrates the chromosomes in the dividing cells of the testis THE TESTIS Connective Tissue Septum These incomplete septae Tunica Albuginea divide the testis into lobes Seminiferous Tubule Interstitial Tissue Loose connective tissue between the seminiferous tubules THE TESTIS Tunica Tunica Albuginea Vasculosa BV Seminiferous Tubule Leydig Cells Blood Vessel (BV) Interstitial Seminiferous Tubule Tissue LEYDIG CELLS Interstitial Tissue BV Seminiferous Tubule NOTE: Leydig cells are endocrine glands and as such are usually located close to blood vessels. These cells are located outside the seminiferous tubules within the loose connective tissue stroma. SEMINIFEROUS TUBULE • Seminiferous Epithelium – Complex Stratified Epithelium consisting of 2 basic cell populations: 1. Sertoli Cells 2. Cells of the Spermatogenic Series: • Spermatogonia • Primary Spermatocyte • Secondary Spermatocyte (Transitory phase: not seen in histological section) • Early Spermatid • Late Spematid SEMINIFEROUS TUBULE Myoid Cell Sertoli Cells Primary Spermatocyte Spermato- gonium Spermato- gonium Lumen Early Spermatids Late Spematids Leydig Cell Spermato- gonium TESTIS AND EPIDIDYMIS • Slide 11 Stain: H&E NOTE: This slide is for ANAT 2020 only Pathway of sperm from point of production to exterior: Seminiferous Tubule Tubuli recti Rete Testes Efferent Ductules Epididymis Vas Deferens Ejaculatory Duct Prostatic Urethra -

Histology of Male Reproductive System
Histology of Male Reproductive System Dr. Rajesh Ranjan Assistant Professor Deptt. of Veterinary Anatomy C.V.Sc, Rewa 1 Male Reproductive System A-Testis B-Epididymis C-Ductus Deferens D-Urethra 1-Pelvic part 2-Penile part E-Penis G-Accessory Glands 1. Seminal vesicles 2-Prostate gland 3-Bulbouretheral gland/ Cowper’s gland Testis The testis remains covered by: Tunica vaginalis- The outermost covering (peritoneal covering of the testis and epididymis). It has a parietal and visceral layer. The parietal layer remains adhered to the scrotum while the visceral layer adheres to the capsule of the testis. The space between the these two layers is called the vaginal cavity. The layers consists of mesothelium lining and connective tissue that blends with the underlying connective tissue of the scrotum. Tunica albuginea: Capsule of the testis Consists of dense irregular connective tissue, predominantly collagen fibers, few elastic fibers and myofibroblast. It has vascular layer (Tunica vasculosa) that contains anatomizing branches of testicular artery and veins. The tunica albuginea gives connective tissue trabeculae called septula testis which converge towards the mediastinum testis. The septula testis divides the testicular parenchyma into number of testicular lobules. Each lobule contains 1-4 seminiferous tubules. Mediastinum testis is a connective tissue area containing the channels of rete testis, large blood and lymph vessels. In bull it occupies the central position along the longitudinal axis of the gonad. Interstitial cells (Leydig cells) The inter-tubular spaces of the testis contain loose C.T., blood and lymph vessels, fibrocytes, free mononuclear cells and interstitial cells called Leydig cells. -

Ta2, Part Iii
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART III Contents: Systemata visceralia Visceral systems Caput V: Systema digestorium Chapter 5: Digestive system Caput VI: Systema respiratorium Chapter 6: Respiratory system Caput VII: Cavitas thoracis Chapter 7: Thoracic cavity Caput VIII: Systema urinarium Chapter 8: Urinary system Caput IX: Systemata genitalia Chapter 9: Genital systems Caput X: Cavitas abdominopelvica Chapter 10: Abdominopelvic cavity Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: SYSTEMA DIGESTORIUM Chapter 5: DIGESTIVE SYSTEM Latin term Latin synonym UK English US English English synonym Other 2772 Systemata visceralia Visceral systems Visceral systems Splanchnologia 2773 Systema digestorium Systema alimentarium Digestive system Digestive system Alimentary system Apparatus digestorius; Gastrointestinal system 2774 Stoma Ostium orale; Os Mouth Mouth 2775 Labia oris Lips Lips See Anatomia generalis (Ch. -

LABORATORY 30 - MALE REPRODUCTIVE SYSTEM - TESTIS and GENITAL DUCTS (First of Two Laboratory Sessions)
LABORATORY 30 - MALE REPRODUCTIVE SYSTEM - TESTIS AND GENITAL DUCTS (first of two laboratory sessions) OBJECTIVES: LIGHT MICROSCOPY: Recognize the duct systems and their components that are found in the testis including: seminiferous tubules, straight tubules, rete testis, efferent ductules and epididymis also spermatic cord with vas deferens. ELECTRON MICROSCOPY: Recognize the components of seminiferous tubules and Leydig cells. ASSIGNMENT FOR TODAY'S LABORATORY GLASS SLIDES SL 156 Testis and epididymis SL 55 Testis and epididymis SL 47 Vas deferens and spermatic cord SL 159 Vas deferens and spermatic cord Trichrome stain POSTED ELECTRON MICROGRAPHS S-98 Seminiferous tubule S-100 Leydig cell Lab 30 Posted EMs; Lab 30 Posted EMs with some yellow labels HISTOLOGY IMAGE REVIEW - available on computers in HSL Chapter 16, Male Reproductive System Frames: 1064-1094 SUPPLEMENTARY ELECTRON MICROGRAPHS Rhodin, J. A.G., An Atlas of Histology Copies of this text are on reserve in the HSL. Male reproductive system, pp. 386 - 398 30 - 1 TESTIS AND GENITAL DUCTS A. TESTIS AND EPIDIDYMIS - Adult. SL 156 (scan) The rough diagram of this slide (below) will help you determine the location of different regions in the section. Refer to J. 21-1 to determine the plane of this section. 1. Identify the tunica albuginea (D) of the testis and seminiferous tubules (C). The triangular piece on this slide is the head of the epididymis (low, med, high) showing the tubules of the epididymis (A) and, to varying extent, tubules of the efferent ductules (ductuli efferentes) (B) (tunica albuginea, red arrows; seminiferous tubules, black line; epididymis, blue line; efferent ductules, green line).