Electrostatics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrostatics Vs Magnetostatics Electrostatics Magnetostatics
Electrostatics vs Magnetostatics Electrostatics Magnetostatics Stationary charges ⇒ Constant Electric Field Steady currents ⇒ Constant Magnetic Field Coulomb’s Law Biot-Savart’s Law 1 ̂ ̂ 4 4 (Inverse Square Law) (Inverse Square Law) Electric field is the negative gradient of the Magnetic field is the curl of magnetic vector electric scalar potential. potential. 1 ′ ′ ′ ′ 4 |′| 4 |′| Electric Scalar Potential Magnetic Vector Potential Three Poisson’s equations for solving Poisson’s equation for solving electric scalar magnetic vector potential potential. Discrete 2 Physical Dipole ′′′ Continuous Magnetic Dipole Moment Electric Dipole Moment 1 1 1 3 ∙̂̂ 3 ∙̂̂ 4 4 Electric field cause by an electric dipole Magnetic field cause by a magnetic dipole Torque on an electric dipole Torque on a magnetic dipole ∙ ∙ Electric force on an electric dipole Magnetic force on a magnetic dipole ∙ ∙ Electric Potential Energy Magnetic Potential Energy of an electric dipole of a magnetic dipole Electric Dipole Moment per unit volume Magnetic Dipole Moment per unit volume (Polarisation) (Magnetisation) ∙ Volume Bound Charge Density Volume Bound Current Density ∙ Surface Bound Charge Density Surface Bound Current Density Volume Charge Density Volume Current Density Net , Free , Bound Net , Free , Bound Volume Charge Volume Current Net , Free , Bound Net ,Free , Bound 1 = Electric field = Magnetic field = Electric Displacement = Auxiliary -

Review of Electrostatics and Magenetostatics
Review of electrostatics and magenetostatics January 12, 2016 1 Electrostatics 1.1 Coulomb’s law and the electric field Starting from Coulomb’s law for the force produced by a charge Q at the origin on a charge q at x, qQ F (x) = 2 x^ 4π0 jxj where x^ is a unit vector pointing from Q toward q. We may generalize this to let the source charge Q be at an arbitrary postion x0 by writing the distance between the charges as jx − x0j and the unit vector from Qto q as x − x0 jx − x0j Then Coulomb’s law becomes qQ x − x0 x − x0 F (x) = 2 0 4π0 jx − xij jx − x j Define the electric field as the force per unit charge at any given position, F (x) E (x) ≡ q Q x − x0 = 3 4π0 jx − x0j We think of the electric field as existing at each point in space, so that any charge q placed at x experiences a force qE (x). Since Coulomb’s law is linear in the charges, the electric field for multiple charges is just the sum of the fields from each, n X qi x − xi E (x) = 4π 3 i=1 0 jx − xij Knowing the electric field is equivalent to knowing Coulomb’s law. To formulate the equivalent of Coulomb’s law for a continuous distribution of charge, we introduce the charge density, ρ (x). We can define this as the total charge per unit volume for a volume centered at the position x, in the limit as the volume becomes “small”. -

We Continue to Compare the Electrostatic and Magnetostatic Fields. the Electrostatic Field Is Conservative
We continue to compare the electrostatic and magnetostatic fields. The electrostatic field is conservative: This allows us to define the potential V: dl because a b is independent of the path. If a vector field has no curl (i.e., is conservative), it must be something's gradient. Gravity is conservative. Therefore you do see water flowing in such a loop without a pump in the physical world. For the magnetic field, If a vector field has no divergence (i.e., is solenoidal), it must be something's curl. In other words, the curl of a vector field has zero divergence. Let’s use another physical context to help you understand this math: Ampère’s law J ds 0 Kirchhoff's current law (KCL) S Since , we can define a vector field A such that Notice that for a given B, A is not unique. For example, if then , because Similarly, for the electrostatic field, the scalar potential V is not unique: If then You have the freedom to choose the reference (Ampère’s law) Going through the math, you will get Here is what means: Just notation. Notice that is a vector. Still remember what means for a scalar field? From a previous lecture: Recall that the choice for A is not unique. It turns out that we can always choose A such that (Ampère’s law) The choice for A is not unique. We choose A such that Here is what means: Notice that is a vector. Thus this is actually three equations: Recall the definition of for a scalar field from a previous lecture: Poisson’s equation for the magnetic field is actually three equations: Compare Poisson’s equation for the magnetic field with that for the electrostatic field: Given J, you can solve A, from which you get B by Given , you can solve V, from which you get E by Exams (Test 2 & Final) problems will not involve the vector potential. -

Roadmap on Transformation Optics 5 Martin Mccall 1,*, John B Pendry 1, Vincenzo Galdi 2, Yun Lai 3, S
Page 1 of 59 AUTHOR SUBMITTED MANUSCRIPT - draft 1 2 3 4 Roadmap on Transformation Optics 5 Martin McCall 1,*, John B Pendry 1, Vincenzo Galdi 2, Yun Lai 3, S. A. R. Horsley 4, Jensen Li 5, Jian 6 Zhu 5, Rhiannon C Mitchell-Thomas 4, Oscar Quevedo-Teruel 6, Philippe Tassin 7, Vincent Ginis 8, 7 9 9 9 6 10 8 Enrica Martini , Gabriele Minatti , Stefano Maci , Mahsa Ebrahimpouri , Yang Hao , Paul Kinsler 11 11,12 13 14 15 9 , Jonathan Gratus , Joseph M Lukens , Andrew M Weiner , Ulf Leonhardt , Igor I. 10 Smolyaninov 16, Vera N. Smolyaninova 17, Robert T. Thompson 18, Martin Wegener 18, Muamer Kadic 11 18 and Steven A. Cummer 19 12 13 14 Affiliations 15 1 16 Imperial College London, Blackett Laboratory, Department of Physics, Prince Consort Road, 17 London SW7 2AZ, United Kingdom 18 19 2 Field & Waves Lab, Department of Engineering, University of Sannio, I-82100 Benevento, Italy 20 21 3 College of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano 22 Science and Technology, Soochow University, Suzhou 215006, China 23 24 4 University of Exeter, Department of Physics and Astronomy, Stocker Road, Exeter, EX4 4QL United 25 26 Kingdom 27 5 28 School of Physics and Astronomy, University of Birmingham, Edgbaston, Birmingham, B15 2TT, 29 United Kingdom 30 31 6 KTH Royal Institute of Technology, SE-10044, Stockholm, Sweden 32 33 7 Department of Physics, Chalmers University , SE-412 96 Göteborg, Sweden 34 35 8 Vrije Universiteit Brussel Pleinlaan 2, 1050 Brussel, Belgium 36 37 9 Dipartimento di Ingegneria dell'Informazione e Scienze Matematiche, University of Siena, Via Roma, 38 39 56 53100 Siena, Italy 40 10 41 School of Electronic Engineering and Computer Science, Queen Mary University of London, 42 London E1 4FZ, United Kingdom 43 44 11 Physics Department, Lancaster University, Lancaster LA1 4 YB, United Kingdom 45 46 12 Cockcroft Institute, Sci-Tech Daresbury, Daresbury WA4 4AD, United Kingdom. -

Lecture 6 Basics of Magnetostatics
Lecture 6 Basics of Magnetostatics Today’s topics 1. New topic – magnetostatics 2. Present a parallel development as we did for electrostatics 3. Derive basic relations from a fundamental force law 4. Derive the analogs of E and G 5. Derive the analog of Gauss’ theorem 6. Derive the integral formulation of magnetostatics 7. Derive the differential form of magnetostatics 8. A few simple problems General comments 1. What is magnetostatics? The study of DC magnetic fields that arise from the motion of charged particles. 2. Since charged particles are present, does this mean we also must have an electric field? No!! Electrons can flow through ions in such a way that current flows but there is still no net charge, and hence no electric field. This is the situation in magnetostatics 3. Magnetostatics is more complicated than electrostatics. 4. Single magnetic charges do not exist in nature. The simplest basic element in magnetostatics is the magnetic dipole, which is equivalent to two, closely paired equal and opposite charges in electrostatics. In magnetostatics the dipole forms from a small circular loop of current. 5. Magnetic geometries are also more complicated than in electrostatics. The vector nature of the magnetic field is less intuitive. 1 The basic force of magnetostatics 1. Recall electrostatics. qq rr = 12 1 2 F12 3 4QF0 rr12 2. In magnetostatics there is an equivalent empirical law which we take as a postulate qq vv×× ¯( rr ) = 12N 0 1212¢± F12 3 4Q rr12 3. The force is proportional to 1/r 2 . = 4. The force is perpendicular to v1 : Fv12¸ 1 0 . -

Electricity and Magnetism
Electricity and Magnetism Definition The Physical phenomena involving electric charges, their motions, and their effects. The motion of a charge is affected by its interaction with the electric field and, for a moving charge, the magnetic field. The electric field acting on a charge arises from the presence of other charges and from a time-varying magnetic field. The magnetic field acting on a moving charge arises from the motion of other charges and from a time-varying electric field. Thus electricity and magnetism are ultimately inextricably linked. In many cases, however, one aspect may dominate, and the separation is meaningful. See also Electric charge; Electric field; Magnetism. History of Electricity From the writings of Thales of Miletus it appears that Westerners knew as long ago as 600 B.C. that amber becomes charged by rubbing. There was little real progress until the English scientist William Gilbert in 1600 described the electrification of many substances and coined the term electricity from the Greek word for amber. As a result, Gilbert is called the father of modern electricity. In 1660 Otto von Guericke invented a crude machine for producing static electricity. It was a ball of sulfur, rotated by a crank with one hand and rubbed with the other. Successors, such as Francis Hauksbee, made improvements that provided experimenters with a ready source of static electricity. Today's highly developed descendant of these early machines is the Van de Graaf generator, which is sometimes used as a particle accelerator. Robert Boyle realized that attraction and repulsion were mutual and that electric force was transmitted through a vacuum (c.1675). -

Chapter 2. Electrostatics
Chapter 2. Electrostatics 2.1. The Electrostatic Field To calculate the force exerted by some electric charges, q1, q2, q3, ... (the source charges) on another charge Q (the test charge) we can use the principle of superposition. This principle states that the interaction between any two charges is completely unaffected by the presence of other charges. The force exerted on Q by q1, q2, and q3 (see Figure 2.1) is therefore equal to the vector sum of the force F1 exerted by q1 on Q, the force F2 exerted by q2 on Q, and the force F3 exerted by q3 on Q. Ftot F1 q3 F3 Q F2 q2 q1 Figure 2.1. Superposition of forces. The force exerted by a charged particle on another charged particle depends on their separation distance, on their velocities and on their accelerations. In this Chapter we will consider the special case in which the source charges are stationary. The electric field produced by stationary source charges is called and electrostatic field. The electric field at a particular point is a vector whose magnitude is proportional to the total force acting on a test charge located at that point, and whose direction is equal to the direction of - 1 - the force acting on a positive test charge. The electric field E , generated by a collection of source charges, is defined as F E = Q where F is the total electric force exerted by the source charges on the test charge Q. It is assumed that the test charge Q is small and therefore does not change the distribution of the source charges. -

Electrostatics and Its Hazards
A Study on Electrostatics and Its Hazards by NIRMALYA BASU (Master of Engineering in Electronics and Telecommunication Engineering) Date of Birth: May 3, 1984 Email: [email protected] Mobile: +919007757186 Address: 162/B/185 Lake Gardens, Kolkata, West Bengal, India, PIN: 700045. Contents Page 1. Introduction 1 2. Electrostatics in Liquids and Its Hazardous Implications 2 2.1. The Phenomenon of Flow Electrification 2 2.2. Hazards in the Industry 5 2.2.1. Metallic Fuel-handling Systems 6 2.2.2. Non-metallic Fuel-handling Systems 6 2.3. Liquid Charging Due to fragmentation and Hazards Arising Thereof 8 2.3.1. The Charging Phenomenon 8 2.3.2. Industrial Hazards 8 2.3.3. Remedial Measures 11 2.4. Standards on Recommended Practices 11 3. Electrostatics in Solids and Its Hazardous Implications 12 3.1. Phenomenon of Contact Charging or Tribocharging of Solids 12 3.1.1. Phenomenon of Charging of Two Metal Objects Brought in Contact with Each Other 12 3.1.2. Phenomenon of Contact Charging of Solids when Insulators are Involved 14 3.1.3. Charging of Two Identical Materials Brought in Contact with Each Other 18 3.1.4. Contact Charging of Granules 21 3.2. Hazards Caused in the Industry Due to Charging of Solids 21 3.2.1. Electrostatic Hazards in Particulate Processes 22 3.2.2. Electrostatic Hazards in the Semiconductor Industry 25 4. Principles of Fire 27 4.1. Combustion 27 4.2. Ignition: Piloted Ignition and Autoignition 27 4.3. Limits of Flammability 28 4.4. Fire Point 29 4.5. -

Electrostatics in Material
Electrostatics in material Bound charges Laplacean methods and dielectrics θ q zˆ ˆ z ˆ η zˆ Z ηˆ PPz= 0 ε 0 σ R E zˆ R s b ε 0 α ε Image methods Separation of variables ε I D top σ Displacement f Q σb < 0 field V r Stored E ε d energy in a dielectric Dbottom σb > 0 −σ above f E ε 0 Dielectric below E ε ε 0 F Forces d ε H Dielectric boundary F conditions 1 x − x xˆ From Physics 212, one might get the impression that going from electrostatics in vacuum to electrostatics in a material is equivalent to replacing epsilon_0 to epsilon > epsilon_0. This is more-or-less true for some dielectric materials such as Class A dielectrics but other types of materials exist. For example, there are permanent electrets which are analogous to permanent magnets. Here the electric field is produced by “bound” charges created by a permanently “frozen-in” polarizability. The polarizability is the electric dipole moment per unit volume that is often induced in the material by an external electric field. We will introduce the displacement field (or D-field) which obeys a Gauss’s Law that only depends on free charges. Free charges are the charges controllable by batteries, currents and the like. To a large extent one cannot totally control bound charges. We discuss the boundary conditions that E-fields and D-fields obey across a dielectric boundary. We turn next to a discussion of Laplace’s Equation in the presence of dielectrics. -

Poisson's Equation in Electrostatics
Poisson’s Equation in Electrostatics Jinn-Liang Liu Institute of Computational and Modeling Science, National Tsing Hua University, Hsinchu 300, Taiwan. E-mail: [email protected] 2007/2/4, 2010, 2011, 2012, 2017 Abstract Poisson’s equation is derived from Coulomb’s law and Gauss’s theorem. It is a par- tial differential equation with broad utility in electrostatics, mechanical engineer- ing, and theoretical physics. It is named after the French mathematician, geometer and physicist Sim´eon-Denis Poisson (1781-1840). Charles Augustin Coulomb (1736- 1806) was a French physicist who discovered an inverse relationship on the force between charges and the square of its distance. Karl Friedrich Gauss (1777-1855) was a German mathematician who also proved the fundamental theorems of algebra and arithmetic. 1 Coulomb’s Law, Electric Field, and Electric Potential Electrostatics is the branch of physics that deals with the forces exerted by a static (i.e. unchanging) electric field upon charged objects [1]. The ba- 19 sic electrical quantity is charge (e = −1.602 × 10− [C] electron charge in coulomb C). In a medium, an isolated charge Q > 0 located at r0 = (x0, y0, z0) produces an electric field E that exerts a force on all other charges. Thus, a charge q > 0 located at a different point r = (x, y, z) experiences a force from Q given by Coulomb’s law [2] as Q r − r0 F = qE = q 2 [N], r = |r − r0| . (1.1) 4πεr |r − r0| A dimension defines some physical characteristics. For example, length [L], mass [M], time [T ], velocity [L/T ], and force [N = ML/T 2] (in newton). -

Active Cloaking and Illusion of Electric Potentials in Electrostatics
Active Cloaking and Illusion of Electric Potentials in Electrostatics Andreas Helfrich-Schkabarenko Esslingen University of Applied Sciences Alik Ismail-Zadeh ( [email protected] ) Karlsruhe Institute of Technology, Institute of Applied Geosciences, Karlsruhe, Germany; Aron Sommer University of Hannover Research Article Keywords: cloaking, self-potentials, inverse theory, numerical modelling, numerical solutions, electric properties Posted Date: January 7th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-140026/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License 1 ACTIVE CLOAKING AND ILLUSION OF ELECTRIC POTENTIALS IN ELECTROSTATICS 2 3 Andreas Helfrich-Schkabarenko1, Alik Ismail-Zadeh2,3, Aron Sommer4 4 5 1 University of Applied Sciences, Esslingen, Germany; 6 [email protected] 7 2 Karlsruhe Institute of Technology, Institute of Applied Geosciences, Karlsruhe, Germany; 8 [email protected] 9 3 Russian Academy of Sciences, Institute of Earthquake Prediction Theory and Mathematical 10 Geophysics, Moscow, Russia; [email protected] 11 4 Leibniz University Hannover, Institute of Information Processing, Hannover, Germany; 12 [email protected] 13 14 Summary - Cloaking and illusion has been demonstrated theoretically and experimentally in 15 several research fields. Here we present for the first time an active exterior cloaking device in 16 electrostatics operating in a two-horizontally-layered electroconductive domain, and use the 17 superposition principle to cloak electric potentials. The device uses an additional current source 18 pattern introduced on the interface between two layers to cancel the total electric potential to 19 be measured. Also, we present an active exterior illusion device allowing for detection of a 20 signal pattern corresponding to any arbitrarily chosen current source instead of the existing 21 current source. -
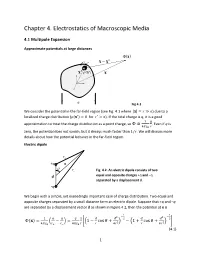
Chapter 4. Electrostatics of Macroscopic Media
Chapter 4. Electrostatics of Macroscopic Media 4.1 Multipole Expansion Approximate potentials at large distances x 3 x x' d x' x' (x') x a Fig 4.1 We consider the potential in the far-field region (see Fig. 4.1 where | | ) due to a localized charge distribution ( for ). If the total charge is q, it is a good approximation to treat the charge distribution as a point charge, so . Even if q is zero, the potential does not vanish, but it decays much faster than . We will discuss more details about how the potential behaves in the far-field region. Electric dipole r+ +q x r- Fig 4.2. An electric dipole consists of two d equal and opposite charges +q and –q separated by a displacement d. -q We begin with a simple, yet exceedingly important case of charge distribution. Two equal and opposite charges separated by a small distance form an electric dipole. Suppose that +q and –q are separated by a displacement vector d as shown in Figure 4.2, then the potential at x is ( ) [( ) ( ) ] (4.1) 1 In the far-field region for | | , (4.2) [( ) ( )] This reduces to the coordinate independent expression (4.3) where is the electric dipole moment. For the dipole p along the z-axis, the electric fields take the form (4.4) { From this, we can obtain the coordinate independent expression (4.5) where is a unit vector. Fig 4.3. Field of an electric dipole 2 Multipole expansion We can expand the potential due to the charge distribution (1.12) ∫ | | using Eq.