Function of Thin Loops of Henle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Kidney Function • Filtration • Reabsorption • Secretion • Excretion • Micturition
About This Chapter • Functions of the kidneys • Anatomy of the urinary system • Overview of kidney function • Filtration • Reabsorption • Secretion • Excretion • Micturition © 2016 Pearson Education, Inc. Functions of the Kidneys • Regulation of extracellular fluid volume and blood pressure • Regulation of osmolarity • Maintenance of ion balance • Homeostatic regulation of pH • Excretion of wastes • Production of hormones © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Kidneys, ureters, bladder, and urethra • Kidneys – Bean-shaped organ – Cortex and medulla © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Functional unit is the nephron – Glomerulus in the Bowman’s capsule – Proximal tubule – The loop of Henle • Descending limb and ascending limb twisted between arterioles forming the juxtaglomerular apparatus – Distal tubule – Collecting ducts © 2016 Pearson Education, Inc. Figure 19.1b Anatomy summary The kidneys are located retroperitoneally at the level of the lower ribs. Inferior Diaphragm vena cava Aorta Left adrenal gland Left kidney Right kidney Renal artery Renal vein Ureter Peritoneum Urinary Rectum (cut) bladder (cut) © 2016 Pearson Education, Inc. Figure 19.1c Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1d Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1f-h Anatomy summary Some nephrons dip deep into the medulla. One nephron has two arterioles and two sets of capillaries that form a portal system. Efferent arteriole Arterioles Peritubular Juxtaglomerular capillaries The cortex apparatus contains all Bowman’s Nephrons Afferent capsules, arteriole Glomerulus proximal Juxtamedullary nephron and distal (capillaries) with vasa recta tubules. Peritubular capillaries Glomerulus The medulla contains loops of Henle and Vasa recta collecting ducts. Collecting duct Loop of Henle © 2016 Pearson Education, Inc. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
![L8-Urine Conc. [PDF]](https://docslib.b-cdn.net/cover/4402/l8-urine-conc-pdf-1384402.webp)
L8-Urine Conc. [PDF]
The loop of Henle is referred to as countercurrent multiplier and vasa recta as countercurrent exchange systems in concentrating and diluting urine. Explain what happens to osmolarity of tubular fluid in the various segments of the loop of Henle when concentrated urine is being produced. Explain the factors that determine the ability of loop of Henle to make a concentrated medullary gradient. Differentiate between water diuresis and osmotic diuresis. Appreciate clinical correlates of diabetes mellitus and diabetes insipidus. Fluid intake The total body water Antidiuretic hormone is controled by : Renal excretion of water Hyperosmolar medullary Changes in the osmolarity of tubular fluid : interstitium 1 2 3 Low osmolarity The osmolarity High osmolarity because of active decrease as it goes up because of the transport of Na+ and because of the reabsorbation of water co-transport of K+ and reabsorption of NaCl Cl- 4 5 Low osmolarity because of High osmolarity because of reabsorption of NaCl , also reabsorption of water in reabsorption of water in present of ADH , present of ADH reabsorption of urea Mechanisms responsible for creation of hyperosmolar medulla: Active Co- Facilitated diffusion transport : transport : diffusion : of : Na+ ions out of the Only of small thick portion of the K+ , Cl- and other amounts of water ascending limb of ions out of the thick from the medullary the loop of henle portion of the Of urea from the tubules into the into the medullary ascending limb of inner medullary medullary interstitium the loop of henle collecting -

Morphology of the Kidney in a Nectarivorous Bird, the Anna's Hummingbird Calypte Anna
J. Zool., Lond. (1998) 244, 175±184 # 1998 The Zoological Society of London Printed in the United Kingdom Morphology of the kidney in a nectarivorous bird, the Anna's hummingbird Calypte anna G. Casotti1*, C. A. Beuchat2 and E. J. Braun3 1 Department of Biology, West Chester University, West Chester, PA, 19383, U.S.A. 2 Department of Biology, San Diego State University, San Diego, CA, 92182, U.S.A. 3 Department of Physiology, Arizona Health Sciences Center, University of Arizona, P.O. Box 245051, Tucson, AZ, 85724±5051, U.S.A. (Accepted 21 April 1997) Abstract The kidneys of Anna's hummingbird (Calypte anna) differ in several signi®cant ways from those of other birds that have been examined. The kidneys of this nectarivore contain very little medullary tissue; 90% of the total volume of the kidneys is cortical tissue, with medulla accounting for only an additional 2%. More than 99% of the nephrons are the so-called `reptilian type', which lack the loop of Henle. The few looped (`mammalian type') nephrons are incorporated into only a few medullary cones per kidney. The loopless nephrons are similar to those of other birds. However, the looped nephrons differ in that they lack the thin descending limb of the loop of Henle, which is found in other birds and is thought to play an important role in the countercurrent multiplier system in the avian kidney. Instead, the cells of the nephron segment following the pars recta of the proximal tubule resemble those of the thick ascending limb, with the large populations of mitochondria that are typical of transporting epithelia and no reduction in cell height. -
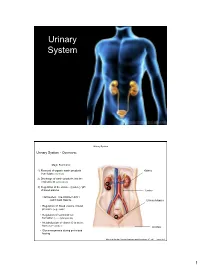
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

Lecture (1) Urinary System
UrinaryUrinaryUrinary systemsystemsystem Dr. Carmen E. Rexach Anatomy 35 Mt. San Antonio College Functions •Storage of urine – Bladder stores up to 1 L of urine • Excretion of urine – Transport of urine out of body • Blood volume regulation – Effects of hormones on kidneys • Regulation of erythrocyte production –Kidneys • Monitor oxygen content of blood • Produce EPO = erthrocyte production Components •Kidneys • Ureters • Urinary Bladder • Urethra Kidneys Gross Anatomy • Kidneys approx weight = 125- 150g each • Retroperitoneal – Anterior surface covered with peritoneum – Posterior surface directly against posterior abdominal wall • Superior surface at about T12 • Inferior surface at about L3 • ureters enter urinary bladder posteriorly • Left kidney 2cm superior to right –Size of liver Transverse section at L1 surface features of kidney • Hilum = the depression along the medial border through which several structures pass –renal artery –renal vein –ureter – renal nerves Surrounding structures • Fibrous capsule – Innermost layer of dense irregular CT – Maintains shape, protection • Adipose capsule (perinephric fat) – Adipose ct of varying thickness – Cushioning and insulation • Renal fascia – Dense irregular CT – Anchors kidney to peritoneum & abdominal wall • Paranephric fat – Outermost, adipose CT between renal fascia and peritoneum Coronal section •Cortex – layer of renal tissue in contact with capsule –Lighter shade –Renal columns= parts of cortex that extend into the medulla between pyramids •Medulla –Innermost – striped due to renal tubules •renal pyramids – 8-15 present in medulla of adult – conical shape – Wide base at corticomedullary junction Coronal section • Renal pelvis – collects from calyces, passes onto ureter •Calyces (pl) – funnel shaped regions – collect urine into pelvis •Minor calyx (s) – in contact with each pyramid •Major calyx (s) – collect from minor Microscopic Anatomy Microscopic anatomy Renal tubules • Nephron – functional unit of the kidney. -

Histology of Urinary System
HISTOLOGY OF URINARY SYSTEM Dr. Rajesh Ranjan Assistant Professor Deptt. of Veterinary Anatomy C.V.Sc. & A.H., Rewa The main organs of this system are: Kidneys Ureters Urinary bladder Urethra KIDNEY Comprises of Capsule Parenchyma Cortex Medulla Cortical labyrinth Medullary rays Outer Medulla Inner Medulla Outer stripes Inner stripes Capsule is made up of collagen fibers, some smooth muscle fibers and blood capillaries. The Parenchyma consists of millions of nephrons, branches of renal arteries, veins, lymphatics and nerves. The Nephrons are the structural and functional unit of kidney. Nephrons can be classified On the basis of location of their glomeruli: Superficial (near the capsule) Mid cortical (near the medulla/Juxtamedullary) On the basis of the length of the loop of henle: Short looped- generally have superficial or mid cortical glomeruli and the tubules extend only into the outer medulla before it reflects back into the cortex. Long looped- have juxtamedullary glomeruli and tubules extend into the inner medulla before reflecting back into the cortex. Nephrons comprises of: 1. Renal corpuscles Glomerulus Glomerular capillaries Mesangiam Bowman’s capsule Parietal layer Visceral layer 2. Proximal tubules Proximal convoluted tubule (PCT) Proximal straight tubule (PST) 3. Henle’s loop Thin descending portion Thin ascending portion Thick ascending portion 4. Distal convoluted tubule 5. Connecting segment 6. Collecting duct Arcade- initial collecting tubule Straight portion- Cortical collecting duct Outer medullary -

The Urinary System Consists of Kidneys, Ureters, Urinary Bladder
Anatomy Lecture Notes Chapter 23 the urinary system consists of kidneys, ureters, urinary bladder, and urethra the function of the kidneys is NOT "to make urine" the kidneys: 1) regulate water balance 2) regular ECF electrolyte levels (Na, K, Ca) 3) eliminate some metabolic wastes urine is a by-product of these functions A. kidneys 1. located against posterior abdominal wall (retroperitoneal) T11 or T12 to L3 right kidney lower than left kidney 2. surrounded by a. pararenal fat (posterior only) b. renal fascia c. adipose capsule - perirenal fat d. renal capsule - dense c.t. covering surface of kidney e. parietal peritoneum Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 3. layers a. cortex - contains renal corpuscles and extends inwards as renal columns b. medulla - consists of renal pyramids which consist mostly of collecting ducts papilla - apex of renal pyramid; where collecting ducts drain into calyx 4. cavities and associated structures a. renal sinus - space in medial part of kidney; contains renal pelvis b. renal pelvis - expanded superior part of ureter minor calyx collects urine from one renal papilla major calyx formed by junction of 2 or more minor calyces renal pelvis formed by junction of all major calyces Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 5. renal hilum - medial indentation; where ureter leaves kidney 6. blood flow through the kidney - renal fraction = 20% of cardiac output aorta renal artery segmental arteries lobar arteries interlobar arteries arcuate arteries cortical radiate (interlobular) arteries afferent arterioles glomerular capillaries (glomerulus) efferent arteriole peritubular capillaries and vasa recta cortical radiate (interlobular) veins arcuate veins interlobar veins renal vein inferior vena cava Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 7. -

Distribution of Calcitonin-Sensitive Adenylate Cyclase Activity Along the Rabbit Kidney Tubule
Proc. Natl. Acad. Sci. USA Vol. 73, No. 10, pp. 3608-3612, October 1976 Cell Biology Distribution of calcitonin-sensitive adenylate cyclase activity along the rabbit kidney tubule (nephron microdissection/enzyme microdetermination) DANIfELLE CHABARDES, MARTINE IMBERT-TEBOUL, MADELEINE MONTfEGUT, ANDRE CLIQUE, AND FRANCOIS MOREL Laboratoire de Physiologie cellulaire, College de France, 11 Place Marcelin Berthelot, Paris 75231, Cedex 05, France Communicated by I. S. Edelman, August 6,1976 ABSTRACT The adenylate cyclase [ATP pyrophosphate- In this paper, we report the distribution of the salmon (SCT) lyase (cyclizing), EC 4.6.1.1] sensitivity to salmon calcitonin in calcitonin sensitive activity of adenylate cyclase. 11 different segments of the rabbit nephron was investigated using a micromethod for enzyme activity measurements in samples, each containing a single piece of tubule. The required MATERIALS AND METHODS segments were isolated by microdissection from collagenase- Methods. The technique used to prepare samples containing treated rabbit kidneys. The results were expressed as femto- one single piece of kidney tubule and to measure their adenylate moles of adenosine 3':5'-cyclic monophosphate formed per mm of tubular length per 30 min of incubation time. In the presence cyclase activity has been reported in detail elsewhere (10). The of 0.1 gg/ml of synthetic salmon calcitonin, it was found that main steps only will briefly be recalled here. The kidneys were eight segments exhibited no hormonal sensitivity whereas removed from pentobarbital anesthetized rabbits (1-2 kg of maximal responses were induced in three segments, the "bright" body weight); pieces of kidney tissue were first incubated in portion of the distal convoluted tubule, the cortical and the "collagenase" solution for 35-45 min at 35°. -

Trace a Urea Molecule Through the Urinary System the Urinary System Chapter 16 – Pages 374-386
VET-114 Animal Anatomy and Physiology 2 Webinar – Chapter 16 Trace a Urea Molecule through the Urinary System The Urinary System Chapter 16 – Pages 374-386 Urinary System Gross Anatomy Figure 16-1, Page 375 • Urology • Kidneys • Ureters • Urinary bladder • Urethra Identify the Structures of the Male Urinary System Bassert Lab Manual – Page 404 Microscopic Anatomy of Kidney • Nephron • 1 million nephrons per kidney Microscopic Anatomy (Histology) of Kidneys • Nephron: basic functional unit of kidneys • Number of nephrons per kidney varies • Each nephron consists of a renal corpuscle, proximal convoluted tubule, loop of Henle and distal convoluted tubule Nephron Structure Figure 16-3, Page 377 • Glomerulus • Bowman’s capsule • Glomerular filtrate • Proximal convoluted tubule (PCT) • Loop of Henle • Distal convoluted tubule (DCT) • Collecting ducts Renal Corpuscle • Located in renal cortex • Function: filters blood in first stage of urine production • Composed of glomerulus surrounded by Bowman’s capsule . Glomerulus: “tuft” of capillaries • Fluid filtered out of blood is called glomerular filtrate Proximal Convoluted Tubule (PCT) • Continuation of capsular space of Bowman’s capsule • Lined with cuboidal epithelial cells with a brush border on lumen side • Twisting path through the cortex • Glomerular filtrate now called the tubular filtrate Loop of Henle • Descends from PCT into medulla, turns, heads upward into cortex • Descending loop has epithelial cells similar to those of PCT • At bottom of loop, epithelial cells flatten to simple squamous epithelial cells and lose their brush border • Ascending loop wall becomes thicker again Distal Convoluted Tubule (DCT) • Continuation of ascending loop of Henle • DCT from all nephrons in the kidney empty into collecting ducts . -

Urinary System
24 The Urinary System nimals living in an aquatic environment face little risk of becoming dehy- 24.1 Overview of the drated. However, animals that started to spend more time on dry land millions Urinary System 941 Aof years ago needed mechanisms to conserve water and prevent dehydration. 24.2 Anatomy of the Kidneys 943 The organ system that performs this function in humans—the urinary system—is the 24.3 Overview of topic of this chapter. The organs of the urinary system are organs of excretion—they Renal Physiology 951 remove wastes and water from the body. Specifically, the urinary system “cleans the 24.4 Renal Physiology I: blood” of metabolic wastes, which are substances produced by the body that it cannot Glomerular Filtration 951 use for any purpose. However, as you will learn in this chapter, the urinary system does 24.5 Renal Physiology II: far more: This system is also essential for removing toxins, maintaining homeostasis of Tubular Reabsorption and Secretion 960 many factors (including blood pH and blood pressure), and producing erythrocytes. 24.6 Renal Physiology III: Read on to discover how the urinary system is vital to your body’s homeostasis. Regulation of Urine Concentration and Volume 968 24.7 Putting It All Together: MODULE 24.1 The Big Picture of Renal Overview of the Urinary System Physiology 974 24.8 Urine and Renal Clearance 974 Learning Outcomes 24.9 Urine Transport, Storage, and Elimination 976 1. List and describe the organs of the urinary system. 2. Describe the major functions of the kidneys. The urinary system is composed of the paired kidneys and the urinary tract.