Bugs R All FINAL Apr 2014 R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquatic Insect and Factors Influencing Their Abundance in Temporary Habitats
Journal of Food Health and Bioenvironmental Science (May - August 2020), 13(2): 17-27 17 Journal of Food Health and Bioenvironmental Science Journal homepage : http://jfhb.dusit.ac.th/ Aquatic Insect and Factors Influencing their Abundance in Temporary Habitats Thanya Reunura & Taeng On Prommi* Department of Biological Science, Faculty of Liberal Arts and Science, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom, 73140 Thailand A r t i c l e i n f o A b s t r a c t Article history: Received : 05 July 2020 Temporary water habitats are usually inhabited by a diverse fauna of aquatic Revised : 10 July 2020 organisms such as aquatic and semiaquatic species and may include rare and Accepted : 19 August 2020 endangered species. In October and November 2016, aquatic insects were sampled Keywords: in selected four temporary sampling sites in Kasetsart University, central Thailand. Temporary Habitat, Aquatic Aquatic D-hand net was used to capture the aquatic insects. Water variables in each Insects, Water Variables habitat were simultaneously measured. A total of 4,820 aquatic insect belonging to 5 orders–Hemiptera (45.119%), Coleoptera (22.51%), Diptera (13.54%), Order Ephemeroptera (10.35%) and Odonata (8.42%) were collected. Eight families were recorded within the Order Hemiptera, with members of Family Notonectidae and the species Anisops bouvieri dominating. Five families were registered within Coleoptera, dominated by family Hydrophilidae, while order Odonata had 2 families dominated by family Libellulidae. Order Diptera was dominated by family Chironomidae. Order Ephemeroptera was dominated by family Baetidae. The values of the Shannon-Weiner index of diversity ranged from 2.118 to 2.487. -

Early Behavioral and Molecular Events Leading to Caste Switching in the Ant Harpegnathos
Downloaded from genesdev.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Early behavioral and molecular events leading to caste switching in the ant Harpegnathos Comzit Opachaloemphan,1,5 Giacomo Mancini,2,5 Nikos Konstantinides,2,5 Apurva Parikh,2 Jakub Mlejnek,2 Hua Yan,1,3,4 Danny Reinberg,1,3,6 and Claude Desplan2,6 1Department of Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, New York 10016, USA; 2Department of Biology, New York University, New York, New York 10003, USA; 3Howard Hughes Medical Institute, New York University School of Medicine, New York, New York 10016, USA Ant societies show a division of labor in which a queen is in charge of reproduction while nonreproductive workers maintain the colony. In Harpegnathos saltator, workers retain reproductive ability, inhibited by the queen phero- mones. Following the queen loss, the colony undergoes social unrest with an antennal dueling tournament. Most workers quickly abandon the tournament while a few workers continue the dueling for months and become gamergates (pseudoqueens). However, the temporal dynamics of the social behavior and molecular mechanisms underlining the caste transition and social dominance remain unclear. By tracking behaviors, we show that the gamergate fate is accurately determined 3 d after initiation of the tournament. To identify genetic factors responsible for this commitment, we compared transcriptomes of different tissues between dueling and nondueling workers. We found that juvenile hormone is globally repressed, whereas ecdysone biosynthesis in the ovary is increased in gamergates. We show that molecular changes in the brain serve as earliest caste predictors compared with other tissues. -

Insects & Spiders of Kanha Tiger Reserve
Some Insects & Spiders of Kanha Tiger Reserve Some by Aniruddha Dhamorikar Insects & Spiders of Kanha Tiger Reserve Aniruddha Dhamorikar 1 2 Study of some Insect orders (Insecta) and Spiders (Arachnida: Araneae) of Kanha Tiger Reserve by The Corbett Foundation Project investigator Aniruddha Dhamorikar Expert advisors Kedar Gore Dr Amol Patwardhan Dr Ashish Tiple Declaration This report is submitted in the fulfillment of the project initiated by The Corbett Foundation under the permission received from the PCCF (Wildlife), Madhya Pradesh, Bhopal, communication code क्रम 車क/ तकनीकी-I / 386 dated January 20, 2014. Kanha Office Admin office Village Baherakhar, P.O. Nikkum 81-88, Atlanta, 8th Floor, 209, Dist Balaghat, Nariman Point, Mumbai, Madhya Pradesh 481116 Maharashtra 400021 Tel.: +91 7636290300 Tel.: +91 22 614666400 [email protected] www.corbettfoundation.org 3 Some Insects and Spiders of Kanha Tiger Reserve by Aniruddha Dhamorikar © The Corbett Foundation. 2015. All rights reserved. No part of this book may be used, reproduced, or transmitted in any form (electronic and in print) for commercial purposes. This book is meant for educational purposes only, and can be reproduced or transmitted electronically or in print with due credit to the author and the publisher. All images are © Aniruddha Dhamorikar unless otherwise mentioned. Image credits (used under Creative Commons): Amol Patwardhan: Mottled emigrant (plate 1.l) Dinesh Valke: Whirligig beetle (plate 10.h) Jeffrey W. Lotz: Kerria lacca (plate 14.o) Piotr Naskrecki, Bud bug (plate 17.e) Beatriz Moisset: Sweat bee (plate 26.h) Lindsay Condon: Mole cricket (plate 28.l) Ashish Tiple: Common hooktail (plate 29.d) Ashish Tiple: Common clubtail (plate 29.e) Aleksandr: Lacewing larva (plate 34.c) Jeff Holman: Flea (plate 35.j) Kosta Mumcuoglu: Louse (plate 35.m) Erturac: Flea (plate 35.n) Cover: Amyciaea forticeps preying on Oecophylla smargdina, with a kleptoparasitic Phorid fly sharing in the meal. -

Observations on Lycaenid Butterflies from Panbari Reserve Forest and Adjoining Areas, Kaziranga, Assam, Northeastern
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 December 2015 | 7(15): 8259–8271 Observations on lycaenid butterflies from Panbari Reserve Forest and adjoining areas, Kaziranga, Assam, northeastern India ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Communication Short Monsoon Jyoti Gogoi OPEN ACCESS Ph.D Student, Department of Ecology & Environmental Science, Assam University, Silchar, Assam 788011, India [email protected] Abstract: A checklist of 116 taxa of Lycaenidae (Blues) along with made to document the Lycaenidae notes on important species in low elevation forest of Panbari Reserve, of Kaziranga-Karbi Hills Kaziranga - West Karbi Hills, upper Assam is reported in this paper based on surveys conducted during 2007–2012 and some recent sightings till date. Important sightings include Blue Gem Poritia Methods erycinoides elsiei, Square-band Brownie Miletis nymphys porus, Plain Plushblue Flos apidanus ahamus, Blue Royal Ancema carmentalis, Study area Elwes Silverline Spindasis elwesi, Artipe skinneri, etc. The Panbari Reserve Forest (26036’N & 93030’E) is protected under the Kaziranga National Park (KNP) Keywords: Butterfly diversity, Kaziranga, Lycaenidae, northeastern India, Panbari Reserve. as its fourth addition (Images 1a,b & 2). The average elevation of the forest is around 90m. The altitude however ranges from 70–300 m. The reserve is very close to National Highway 37 (NH37) on the Guwahati- The Lycaenidae (Blues) butterfly diversity in low Jorhat route. The reserve falls between Golaghat and elevation forests of Panbari Reserve, Kaziranga - West Karb Anglong (KA) districts of Assam. To the north of Karbi Hills, upper Assam is reported in this paper. Karbi the reserve lies Dollamora proposed reserve in Karbi Hills constitue a chain of hill ranges lying in middle Assam Anglong District and on the southern boundary is a in the southern bank of the river Brahmaputra. -

The Superfamily Calopterygoidea in South China: Taxonomy and Distribution. Progress Report for 2009 Surveys Zhang Haomiao* *PH D
International Dragonfly Fund - Report 26 (2010): 1-36 1 The Superfamily Calopterygoidea in South China: taxonomy and distribution. Progress Report for 2009 surveys Zhang Haomiao* *PH D student at the Department of Entomology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China. Email: [email protected] Introduction Three families in the superfamily Calopterygoidea occur in China, viz. the Calo- pterygidae, Chlorocyphidae and Euphaeidae. They include numerous species that are distributed widely across South China, mainly in streams and upland running waters at moderate altitudes. To date, our knowledge of Chinese spe- cies has remained inadequate: the taxonomy of some genera is unresolved and no attempt has been made to map the distribution of the various species and genera. This project is therefore aimed at providing taxonomic (including on larval morphology), biological, and distributional information on the super- family in South China. In 2009, two series of surveys were conducted to Southwest China-Guizhou and Yunnan Provinces. The two provinces are characterized by karst limestone arranged in steep hills and intermontane basins. The climate is warm and the weather is frequently cloudy and rainy all year. This area is usually regarded as one of biodiversity “hotspot” in China (Xu & Wilkes, 2004). Many interesting species are recorded, the checklist and photos of these sur- veys are reported here. And the progress of the research on the superfamily Calopterygoidea is appended. Methods Odonata were recorded by the specimens collected and identified from pho- tographs. The working team includes only four people, the surveys to South- west China were completed by the author and the photographer, Mr. -

A Preliminary Check List of Odonates from Calicut University Campus, Calicut, Kerala, South India
Journal of Entomology and Zoology Studies 2015; 3 (2): 260-263 E-ISSN: 2320-7078 P-ISSN: 2349-6800 A preliminary check list of Odonates from Calicut JEZS 2015; 3 (2): 260-263 university campus, Calicut, Kerala, South India © 2015 JEZS Received: 20-02-2015 Accepted: 04-03-2015 Jisha Krishnan E. K, Sebastian C. D. Jisha Krishnan E. K Abstract Molecular Biology Laboratory, Dragonflies and damselflies, collectively called odonates, are one of the most common insects flying Department of Zoology, over forest, fields, meadows, ponds and rivers. Approximately 6500 extant species in over 600 genera University of Calicut, Kerala, and 28 families are known all over the world. About 474 species in 142 genera and 18 families are 673 635 India. identified from India, out of which 154 species are from Kerala. Here we developed a preliminary Sebastian C. D. checklist of Odonata populations found in Calicut University Campus. The study revealed 27 species Department of Zoology, coming under 4 families and 21 genera. Suborder Anisoptera (dragonflies) were represented by the University of Calicut, Kerala, family Libellulidae, Aeshnidae and Gomphidae while the suborder Zygoptera by the family 673 635 India. Coenagrionidae. The two dominant familes of Odonates – Libellulidae and Coenagrionidae – were found to exist in all habitats under the study. Keywords: Odonata, Calicut University Camps, checklist 1. Introduction Odonata are a striking aquatic and aerial component of environment in terms of both biomass and their influence as predators [1]. These attributes have prompted studies of odonate life histories, behavior, and diet [2]. The fossil record of these species dates back to carboniferous period over 350 million years ago. -

Pacific Islands Area
Habitat Planting for Pollinators Pacific Islands Area November 2014 The Xerces Society for Invertebrate Conservation www.xerces.org Acknowledgements This document is the result of collaboration with state and federal agencies and educational institutions. The authors would like to express their sincere gratitude for the technical assistance and time spent suggesting, advising, reviewing, and editing. In particular, we would like to thank the staff at the Hoolehua Plant Materials Center on the Hawaiian Island of Molokai, NRCS staff in Hawaii and American Samoa, and researchers and extension personnel at American Samoa Community College Land Grant (especially Mark Schmaedick). Authors Written by Jolie Goldenetz-Dollar (American Samoa Community College), Brianna Borders, Eric Lee- Mäder, and Mace Vaughan (The Xerces Society for Invertebrate Conservation), and Gregory Koob, Kawika Duvauchelle, and Glenn Sakamoto (USDA Natural Resources Conservation Service). Editing and layout Ashley Minnerath (The Xerces Society). Updated November 2014 by Sara Morris, Emily Krafft, and Anne Stine (The Xerces Society). Photographs We thank the photographers who generously allowed use of their images. Copyright of all photographs remains with the photographers. Cover main: Jolie Goldenetz-Dollar, American Samoa Community College. Cover bottom left: John Kaia, Lahaina Photography. Cover bottom right: Gregory Koob, Hawaii Natural Resources Conservation Service. Funding This technical note was funded by the U.S. Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS) and produced jointly by the NRCS and The Xerces Society for Invertebrate Conservation. Additional support was provided by the National Institute for Food and Agriculture (USDA). Please contact Tony Ingersoll ([email protected]) for more information about this publication. -

Forestry Department Food and Agriculture Organization of the United Nations
Forestry Department Food and Agriculture Organization of the United Nations Forest Health & Biosecurity Working Papers OVERVIEW OF FOREST PESTS INDONESIA January 2007 Forest Resources Development Service Working Paper FBS/19E Forest Management Division FAO, Rome, Italy Forestry Department Overview of forest pests - Indonesia DISCLAIMER The aim of this document is to give an overview of the forest pest1 situation in Indonesia. It is not intended to be a comprehensive review. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. © FAO 2007 1 Pest: Any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products (FAO, 2004). ii Overview of forest pests - Indonesia TABLE OF CONTENTS Introduction..................................................................................................................... 1 Forest pests...................................................................................................................... 1 Naturally regenerating forests..................................................................................... 1 Insects ..................................................................................................................... 1 Diseases.................................................................................................................. -
![Pacific Islands: Their Culture, (Syzygium Cumini) [Negative Impacts on Cultivation of Bananas] Environment, and Use](https://docslib.b-cdn.net/cover/3242/pacific-islands-their-culture-syzygium-cumini-negative-impacts-on-cultivation-of-bananas-environment-and-use-503242.webp)
Pacific Islands: Their Culture, (Syzygium Cumini) [Negative Impacts on Cultivation of Bananas] Environment, and Use
Family: Myrtaceae Taxon: Syzygium cumini Synonym: Eugenia cumini (L.) Druce Common Name jambolan Eugenia jambolana Lam. Malabar plum Myrtus cumini L. (basionym) jamélongue Syzygium jambolanum (Lam.) DC. Jambolanapflaume Caryophyllus jambos Stokes guayabo pesgua Java plum Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(Hawai'i) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 9 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see y Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or burrs -

76179-Sfs English Med School
BASIC DETAIL 76179-S F S ENGLISH MED SCHOOL KANNUR KERALA Dated : 21/08/2019 S F S ENGLISH MED SCHOOL KANNUR SCHOOL CODE 76179 SCHOOL NAME KERALA S F S ENGLISH MEDIUM AFFILIATION ADDRESS SCHOOL,VETTIAPPALLY 931202 CODE VAYAL,THANNA P O PRINCIPAL'S PRINCIPAL MR JAISMON FRANCIS CONTACT 9481404007 NUMBER PRINCIPAL'S PRINCIPAL'S EMAIL ID [email protected] RETIREMENT 15/11/2039 DATE SCHOOL'S CONTACT SCHOOL'S 0497-2734985 [email protected] NUMBER EMAIL ID SCHOOL'S FAX SCHOOL'S WEBSITE www.sfsschoolknr.com 04972734985 NUMBER YEAR OF LANDMARK NEAR SCHOOL N/R Andathode Madrasa 2003 ESTABLISHMENT AFFILIATION AFFILIATION VALIDITY 2017 TO 2022 PROVISIONAL STATUS NAME OF THE Kerala Fransalian REGISTRATION TRUST/SOCIETY/COMPANY 29/06/1973 Educational Society DATE REGISTERED WITH SOCIETY REGISTRATION REGISTRATION PERMANENT Education S.No.K.31 NUMBER VALIDITY REGISTRATION General Education(N) NOC ISSUING NOC ISSUING AUTHORITY 31/07/2012 Department, Govt of Kerala DATE NON PROPRIETY CHARACTER NO OBJECTION AFFIDAVIT/NON VIEW (1) VIEW (2) CERTIFICATE PROFIT COMPANY AFFIDAVIT FACULTY DETAILS 76179--S F S ENGLISH MED SCHOOL KANNUR KERALA Dated: 21/08/2019 TOTAL NUMBER OF TEACHERS (ALL CLASSES) 26 NUMBER OF PGTs 6 NUMBER OF TGTs 4 NUMBER OF PRTs 12 NUMBER OF PETs 1 OTHER NON-TEACHING STAFF 7 NUMBER OF MANDATORY TRAINING QUALIFIED NUMBER OF TRAININGS ATTENDED BY 15 5 TEACHERS FACULTY SINCE LAST YEAR WHETHER COUNSELLOR AND WELLNESS WHETHER SPECIAL EDUCATOR APPOINTED? TEACHER APPOINTED? HAS MANDATORY TRAINING OF TEACHERS AS PER THE TRAINING POLICY (SECTION-16 -

Zoo Og Cal S Ryey of I Dia
M l CELLANEOUS P BLI ATIO~ OCCA (0 AL PAPER O. 20 I ecords of the Zoo og cal S ryey of I dia FIELD ECOLOGY, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By ARUN KUMAR AND MAHA8IR PRASAD Issued by the Director Zoo1ogical Survey of India, Calcutta RECORDS OFTHE Zoological Survey of India MISCELLANEOUS PUBLICATION OCCASIONAL PAPER NO. 20 FIELD ECOLOGY, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By AruD Kumar and Mababir Prasad Northern Regional Station, Zoological Survey of Inelia, Dellra D,u, Edited by the Director, Zoological Survey of India, ('a/cult" 1981 © Copyright 1981. Government of India Published in March, 1981 PRICE: Inland: Rs. 40.00 Foreign: £ 4.50 $ 12.00 Printed in Indi~ at SAAKHHAR MUDRAN 4 Deshapran Shasmal Road Calcutta 700 033 and Published by the Controller of Publications. Civil Lines, Delhi 110006 RECORDS OFTHE Zoological Survey of India MISCELLANEOUS PUBLICATION Occasional Paper No. 20 1981 Pages 1-118 CONTENTS Page No. INTRODUCTION 1 GEOGRAPHICAL FEATURES, DIVISIONS AND CLIMATE OF WESTERN HIMALAYA 4 BRIEF DESCRIPTION OF TYPICAL OOONATA BIOTOPES IN WESTERN HIMALAYA 5 PHENOLOGY 8 KEY TO THE ODONATA OF WESTERN HIMALAYA 9 CHECK-LIST OF OOONATA OF WESTERN HIMALAYA WITH NOTES ON FIELD ECOLOGY 32 ZOOGEOGRAPHY OF ODONATA OF WESTERN HIMALAYA 67 SUMMARY 72 REFERENCES 98 FIELD ECOLOGV, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By ARUN KUMAR AND MAHABIR PRASAD": Northern Regional Station, Zoological Survey of India, Dehra Dun (With 13 Text figures, 1 Plate and 3 Tables) INTRODUCTION Within the Indian sub-region, the Odonata Fauna of Himalaya has so far been studied most extensively. -
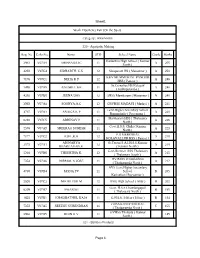
Sheet1 Page 1 Work Experience Fair (On the Spot) Reg. No Code No
Sheet1 Work Experience Fair (On the Spot) Category : HSS/VHSS 320 - Agarbathi Making Reg. No Code No. Name STD School Name Grade Marks Kadachira High School ( Kannur 2961 V07A8 SHINSARAJ C 11 A 255 South ) 4293 V07C4 SIDHARTH. C.K 12 Sivapuram HS ( Mattannur ) A 250 KKV MEMMORIAL PANOOR 7176 V07C1 NEHA K P 12 A 246 HSS ( Panoor ) St.Cornelius HS Kolayad 3490 V07B9 ANUSREE KR 11 A 240 ( Kuthuparamba ) 4292 V07B8 JEENA DAS 12 GHSS Mambaram ( Mattannur ) A 240 3963 V07A4 SOORYA.A.C 12 GBVHSS MADAYI ( Madayi ) A 231 Govt.Higher Secondary School 4767 V07B3 ASHIGA K P 11 A 228 Ramanthally ( Payyannur ) Mambaram HSS ( Thalassery 6210 V07C5 ABHINAV P 11 A 226 North ) Govt.H.S.S. Chala ( Kannur 2576 V07A9 SREERAG SURESH 11 A 223 North ) P.R MEMORIAL 7177 V07C2 RIJIL.K.K 12 A 218 KOLAVALLUR HSS ( Panoor ) AISWARYA St.Teresa`S A.I.H.S.S.Kannur 2575 V07A1 12 A 218 BHARGAVAN.K ( Kannur North ) Govt.Brennen HSS Thalassery 5204 V07B6 THEERTHA K. 12 A 215 ( Thalassery South ) BVJMHS Perumbadavu 7354 V07A6 NIRMAL K JOSE 11 A 212 ( Thalparamba North ) AVS Govt.Higher Secondary 4768 V07B4 JASNA PV 11 School B 205 Karivellur ( Payyannur ) 2058 V07C3 NIKHILESH M 12 Iritty High School ( Iritty ) B 202 Govt. H.S.S Chundangapoil 6209 V07B7 SWARAG 11 B 185 ( Thalassery North ) 1625 V07B1 KHADIJATHUL IJAJA 11 G.H.S.S. Irikkur ( Irikur ) B 184 GHSS KANIYANCHAL 7353 V07A5 SEETHU SURENDRAN 11 C 155 ( Thalparamba North ) GVHSS Thottada ( Kannur 2962 V07B5 RIJIN K V 11 145 South ) 321 - Bamboo Products Page 1 Sheet1 Reg.