Coker Distillate(S)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upgrading of Coker Distillate Under Variable Hydrotreating Operating Conditions
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Egyptian Journal of Petroleum (2011) 20, 25–31 Egyptian Petroleum Research Institute Egyptian Journal of Petroleum www.elsevier.com/locate/egyjp www.sciencedirect.com Upgrading of coker distillate under variable hydrotreating operating conditions H.A. Elsayed *, H.S. Ahmed, M.F. Monufy Refining Department, Egyptian Research Institute, Nasr City, Cairo, Egypt Received 28 February 2010; accepted 2 January 2011 Available online 19 October 2011 KEYWORDS Abstract Studies on hydrotreating coker distillates, produced from a delayed coker unit were done Hydrotreating; using a commercially available CoMo/c-Al2O3 catalyst, on which 0.2 wt% P2O5 was added in order Hydrotreating catalyst; to improve its characteristics. The experimental studies were conducted in a fixed-bed continuous- Coker distillate; reactor (cata-test unit) at temperatures (300–400 °C) and total hydrogen pressure (40–65 bar). These Hydrodesulfurization conditions have affected the feedstock characteristics and great reduction of sulfur, aromatics and boiling ranges. Other improvements were obtained in diesel index (DI) due to hydrogenation reac- tion of aromatics and desulfurization of its sulfur contents. ª 2011 Egyptian Petroleum Research Institute. Production and hosting by Elsevier B.V. Open access under CC BY-NC-ND license. 1. Introduction in fuels and gas oil or diesel to <0.01 wt%. The removal of sulfur containing compounds, such as thiophenes, mercaptans, In recent years, sulfur reduction from light gas oil or diesel fuel dibenzothiophene and especially its alkyl substituted deriva- using catalytic hydrodesulfurization (HDS) is a routine opera- tives is necessary in order to minimize the polluting effects of tion in petroleum refining process due to environmental regu- containing gases such as SO2 that may be released into the lations and to improve the combustion qualities of automotive atmosphere during combustion [1]. -

Residuum Processing
CHAPTER SIX Residuum Processing DELAYED COKING Delayed coking is a thermal process in which the vacuum residue from crude distillation is heated in a furnace then confined in a reaction zone or coke drum under proper operating conditions of temperature and pressure until the unvaporized portion of the furnace effluent is converted to vapor and coke. Delayed coking is an endothermic reaction, with the furnace supplying the necessary heat for the coking reactions. The reactions in the delayed coking are complex. In the initial phase, the feed is partially vaporized and cracked as it passes through the furnace. In the next step, cracking of the vapor occurs as it passes through the drum. In the final step, succes- sive cracking and polymerization of the liquid confined in the drum takes place at high temperatures, until the liquid is converted into vapor and coke. The coke produced in the delayed coker is almost pure carbon contain- ing some of the impurities of the feed, such as sulfur and metals. THE DELAYED COKING PROCESS The reduced crude or vacuum resid enters the coke fractionator bottom surge zone (see Figure 6-1). The feed is mixed with recycle condensed in the bottom section of the fractionator and pumped by heater charge pump P-04 through coke heater H-Ol, where the charge is rapidly heated to the desired temperature for coke formation in the coke drums. Steam is injected in each heater coil to maintain the required minimum velocity and residence time and suppress the formation of coke in the heater coils. BLOWDOWN CONDENSER BLOWDOWN SETTLING DRUM V-05 E-02 TO FLARE BLOWDOWN DECOKING WATER BLOWDOWN WATER SLOP OIL STORAGETANK CIRCULATION PUMP PUMP T-01 P-08 OIL COOLER P-09 SLOP OIL E-01 COKER RAW WATER BLOWDOW^ DRUM V-04 COKER GAS FRACTIONATOR N. -

Environmental, Health, and Safety Guidelines for Petroleum Refining
ENVIRONMENTAL, HEALTH, AND SAFETY GUIDELINES PETROLEUM REFINING November 17, 2016 ENVIRONMENTAL, HEALTH, AND SAFETY GUIDELINES FOR PETROLEUM REFINING INTRODUCTION 1. The Environmental, Health, and Safety (EHS) Guidelines are technical reference documents with general and industry-specific examples of Good International Industry Practice (GIIP).1 When one or more members of the World Bank Group are involved in a project, these EHS Guidelines are applied as required by their respective policies and standards. These industry sector EHS Guidelines are designed to be used together with the General EHS Guidelines document, which provides guidance to users on common EHS issues potentially applicable to all industry sectors. For complex projects, use of multiple industry sector guidelines may be necessary. A complete list of industry sector guidelines can be found at: www.ifc.org/ehsguidelines. 2. The EHS Guidelines contain the performance levels and measures that are generally considered to be achievable in new facilities by existing technology at reasonable costs. Application of the EHS Guidelines to existing facilities may involve the establishment of site-specific targets, with an appropriate timetable for achieving them. 3. The applicability of the EHS Guidelines should be tailored to the hazards and risks established for each project on the basis of the results of an environmental assessment in which site-specific variables— such as host country context, assimilative capacity of the environment, and other project factors—are taken into account. The applicability of specific technical recommendations should be based on the professional opinion of qualified and experienced persons. 4. When host country regulations differ from the levels and measures presented in the EHS Guidelines, projects are expected to achieve whichever is more stringent. -

Petroleum Residue Upgrading Via Delayed Coking: a Review
Petroleum Residue Upgrading Via Delayed Coking: A Review Ashish N. Sawarkar, Aniruddha B. Pandit, Shriniwas D. Samant and Jyeshtharaj B. Joshi* Institute of Chemical Technology, University of Mumbai, N. P. Marg, Matunga, Mumbai, Maharashtra, 400019, India World petroleum residue processing capacity has reached about 725 million metric tons per annum (MMTPA). The high demand for transportation fuels and the ever-rising heavy nature of crude oil have resulted in a renewed interest in the bottom-of-the-barrel processing using various conver- sion processes. Delayed coking, known for processing virtually any refi nery stream (which not only poses a serious threat to environment, but also involves a disposal cost) has garnered tremendous importance in the current refi ning scenario. Needle coke obtained from delayed coking process is a highly sought-after product, which is used in electric arc furnaces (in the form of graphite electrodes) in steel making applications. In the present communication, the published literature has been extensively analyzed and a state-of-the-art review has been written that includes: (1) importance and place of delayed coking as a residue upgrading process in the current refi ning scenario; (2) coking mechanism and kinetics; (3) design aspects; (4) feedstocks suitable for the production of needle coke; (5) characteristics of needle coke; (6) factors affecting needle coke quality and quantity; and (7) future market for needle coke. An attempt has been made to get the above-mentioned aspects together in a coherent theme so that the information is available at a glance and could be of signifi cant use for researchers and practising refi ners. -

Refinery Scheme's Mass Targeting and Bottom
REFINERY SCHEME’S MASS TARGETING AND BOTTOM SECTION SYNTHESIS FOR HEAVY OIL A Thesis by ABDULAZIZ ABDULKAREEM A ALNAJJAR Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Mahmoud M.El-Halwagi Committee Members, M. Sam Mannan Sergiy Butenko Head of Department, M. Nazmul Karim December 2015 Major Subject: Chemical Engineering Copyright 2015 Abdulaziz A. Alnajjar ABSTRACT This work evaluates the introduction of heavy oil in a refinery as a first step. The first step will yield an increase in the production of the bottom products (vacuum residue, gas oil and diesel). It will also reduce the production of the light products (gases, LPG and naphtha) from ADU/VDU for oil with API above 20. However, we showed that if the heavy oil is below 20 API, the vacuum residue will be the only increasing product. This also reflects on the unit capital cost. The power and steam required by the refinery should also increase as crude oil becomes heavier due to the high amount of steam used in the delayed coker unit. Nevertheless, the fuel for the fire heaters does not show the expected change as compared to the model. The report goes to a further step by replacing bottom product processes with gasification and syngas routes. This step results to reduce the total production of fuel. Therefore, the fuel gasification paths MTG, DME (direct and indirect), and FT are more valuable than other gasification paths. All fuel paths showed a similar amount of fuel production, yielding extra production around 100,000 lb/hr compared to the base case. -

Petroleum Refining Water/Wastewater Management
Petroleum refining Operations Best Practice water/wastewater Series use and management 2010 www.ipieca.org The global oil and gas industry association for environmental and social issues 5th Floor, 209–215 Blackfriars Road, London SE1 8NL, United Kingdom Telephone: +44 (0)20 7633 2388 Facsimile: +44 (0)20 7633 2389 E-mail: [email protected] Internet: www.ipieca.org © IPIECA 2010 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior consent of IPIECA. This publication is printed on paper manufactured from fibre obtained from sustainably grown softwood forests and bleached without any damage to the environment. Petroleum refining water/wastewater use and management IPIECA Operations Best Practice Series This document was prepared by AECOM, Inc. on behalf of the IPIECA Refinery Water Management Task Force. The assistance of M. Venkatesh of ENSR–AECOM is gratefully acknowledged. Cover photographs reproduced courtesy of the following (clockwise from top left): ExxonMobil; Nexen; Photodisc Inc.; iStockphoto; Corbis; Shutterstock.com. IPIECA Contents Introduction 2 Effluent treatment 25 Process wastewater pretreatment 25 Refinery water overview 3 Desalter effluent treatment 25 Overall refinery water balance 3 Wastewater segregation 27 Sources of water 3 Primary treatment 28 Water leaving the refinery 4 First stage: separation Raw water treatment: 5 (oil/water separators, API separators) -

Volume 1: Section 3
Volume 1: Project Description TOTAL Upgrader Project Section 3: Project Description 3 Project Description 3.1 Introduction This section provides the following information about the TOTAL Upgrader: • a process description • mass and energy balances, measurement and required materials • the estimated quantities and properties of the upgrader’s products and byproducts along with an overview of air emissions and mitigation measures • the plot plan for the upgrader and the consideration given during design to safety, environmental protection and equipment integrity • the utilities and offsites required to support the upgrader • alternative design considerations 3.2 Process Description After an extensive evaluation process, TOTAL has selected an upgrader configuration with the following components: • distillation: • diluent recovery unit (DRU) • vacuum distillation unit (VDU) – Phase 2 • coking: • delayed coker unit (DCU) • unsaturated gas plant (USGP) • hydrotreatment and desulphurization: • naphtha hydrotreater (NHT) • distillate hydrotreater (DHT) • mild hydrocracker (MHC) – Phase 2 • hydrogen production units • saturated gas plant (SGP) • amine regeneration unit (ARU) • sour water stripping units (SWS) • sulphur recovery units (SRU) including a tail gas treating unit (TGTU) For an overview of the process, see Figure 3.2-1. For an evaluation of alternatives to this configuration, see Section 3.7. The upgrader will be developed in two phases. All the process units listed will be constructed in Phase 1 except for those identified to be constructed in Phase 2. The VDU, MHC and additional components of the DRU, DCU, ARU, hydrogen unit and SRU will be constructed in Phase 2. For information on the site construction sequence, see Section 4.3.6.1 and Table 4.3-1. -

WO 2016/015045 Al 28 January 2016 (28.01.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/015045 Al 28 January 2016 (28.01.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every CIOG 27/04 (2006.01) CIOG 53/14 (2006.01) kind of national protection available): AE, AG, AL, AM, CIOG 27/14 (2006.01) CIOG 55/04 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, CIOG 31/06 (2006.01) CIOG 21/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, CIOG 53/04 (2006.01) CIOB 55/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US20 15/042234 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 27 July 2015 (27.07.2015) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/028,892 25 July 2014 (25.07.2014) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: SAUDI ARABIAN OIL COMPANY DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, [SA/SA]; Box 5000, Dhahran, 3 13 11 (SA). -

Delayed Coker Environmental Assessment Registration
Delayed Coker Environmental Assessment Registration REGISTRATION PURSUANT TO SECTION 40(1) AND 45 (1) OF THE ENVIRONMENTAL ASSESSMENT REGULATIONS, 2003 UNDER THE ENVIRONMENTAL PROTECTION ACT (SNL 2002 CE-14.2) FOR THE PROPOSED NARL FUELS LP DEALYED COKER PROJECT JULY 2019 Submitted to: Minister of Municipal Affairs and Environment P. O Box 8700 St. John's NF A1B 4J6 Attention: Director of Environmental Assessment Submitted by: NARL Fuels LP P.O. Box 40 Come By Chance, NL A0B 1N0 Print Date July 5, 2019 DELAYED COKER PROJECT Executive Summary This document provides the Project Registration for the construction and operation of a Delayed Coker complex equipped with a small integrated Crude Oil Fractionation Unit, a Distillate Hydrotreater, and assorted utilities. NARL Fuels LP, a fully owned subsidiary of North Atlantic Refining Ltd. Refinery (“NARL”), proposes to construct and operate this facility in Come by Chance, northwest of the existing NARL refinery. Effective, January 1, 2020, the International Maritime Organization (IMO) will implement a global cap of 0.5% sulfur on fuel oil used by all ocean-going vessels. As a result, existing markets for residual fuel oil higher than 0.5% sulfur are predicted to experience significant declines. Combined with the predicted increases in heavy sour and medium sour crude production, this will require an estimated three million barrels per day of additional Delayed Coker capacity to be built over the next twenty years worldwide. Operation of the Delayed Coker will consume vacuum residue from the adjacent NARL Refinery allowing for the shutdown of its Visbreaking Unit. The Integrated Crude Fractionation unit will operate on crude sourced from offshore Newfoundland and elsewhere. -

Environmental, Health, and Safety Guidelines for Petroleum Refining
This document is NO LONGER IN USE by the World Bank Group. The new version of the World Bank Group Environmental, Health and Safety Guidelines are available at http://www.ifc.org/ehsguidelines Environmental, Health, and Safety Guidelines PETROLEUM REFINING RAFT FOR ECOND UBLIC ONSULTATION MARCH D S P C — 2016 WORLD BANK GROUP Environmental, Health, and Safety Guidelines for Petroleum Refining Introduction 1. The Environmental, Health, and Safety (EHS) Guidelines are technical reference documents with general and industry-specific examples of Good International Industry Practice (GIIP)1. When one or more members of the World Bank Group are involved in a project, these EHS Guidelines are applied as required by their respective policies and standards. These industry sector EHS guidelines are designed to be used together with the General EHS Guidelines document, which provides guidance to users on common EHS issues potentially applicable to all industry sectors. For complex projects, use of multiple industry sector guidelines may be necessary. A complete list of industry-sector guidelines can be found at: www.ifc.org/ehsguidelines 2. The EHS Guidelines contain the performance levels and measures that are generally considered to be achievable in new facilities by existing technology at reasonable costs. Application of the EHS Guidelines to existing facilities may involve the establishment of site-specific targets, with an appropriate timetable for achieving them. 3. The applicability of the EHS Guidelines should be tailored to the hazards and risks established for each project on the basis of the results of an environmental assessment in which site-specific variables, such as host country context, assimilative capacity of the environment, and other project factors, are taken into account. -

Refinery Intermediate Product Literature Review Final Report
Refinery Intermediate Product Literature Review TCEQ PGA No. 582-13-30089-FY14-15 TCEQ Tracking No. 2014-22-PCR# 42253 Final Report Prepared for Texas Commission on Environmental Quality Mr. Mark Muldoon, Project Manager Air Quality Division [email protected] 512.239.4895 Prepared by Kirsten Rosselot, PE [email protected] 818.878.0454 Vincent M. Torres, MSE, PE, MAC [email protected] 512.471.5803 The University of Texas at Austin Center for Energy & Environmental Resources 10100 Burnet Rd., Bldg. 133 Austin, TX 78758 August 2014 - Refinery Intermediate Product Literature Review Project: Final Report - Contents Contents ......................................................................................................................................................... i List of acronyms and abbreviations ............................................................................................................. iii Introduction .................................................................................................................................................. 1 Intermediate streams ................................................................................................................................... 3 Useful formulas ......................................................................................................................................... 4 Literature ............................................................................................................................................... -
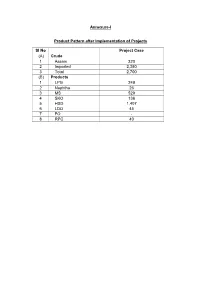
Annexure-I Product Pattern After Implementation of Projects Sl No
Annexure-I Product Pattern after Implementation of Projects Sl No Project Case (A) Crude 1 Assam 320 2 Imported 2,380 3 Total 2,700 (B) Products 1 LPG 249 2 Naphtha 26 3 MS 529 4 SKO 136 5 HSD 1,407 6 LDO 45 7 FO - 8 RPC 40 Annexure-II Fresh Water Requirement Sl Particulars Source Requirement Remarks No. (m3/hr) (1) Raw water for - - No additional raw water CDU-II, required DHDT, SDS & CRU-MSQ (2) Raw water for Ground water 50 Treated effluent water from INDMAX TTP will be reused in Cooling Tower make-up. Raw water requirement is for Service Water and Drinking Water only. Annexure-III Utilities Requirement (Steam, Power & Fuel) Sl Particulars Unit CDU-II DHDT CRU- INDMAX IGHDS SDS Total No. MSQ FCC Unit Unit Unit (1) Steam MT/hr No - Net SL Steam No No No 4.5 - change 4.5 - Net MP Steam change change change 34.4 8.8 43.2 - Net HP Steam (0.3) - (0.3) (2) Power KW - 300 - 3,620 255 45 4,220 (3) Fuel (Fuel Gas) MMKcal/hr 0.63 0.44 0.17 - 0.9 - 0.9 Note: There is no direct fuel requirement in INDMAX Unit and SDS Unit Annexure-IV Stack Emissions Sl Stack Emissions (kg/hr) No. SOx NOx (1) INDMAX FCC Regenerator 43.6 16.1 (2) IGHDS Unit 0.04 0.16 (3) CDU-II 6.86 0.55 (4) DHDT 0.03 0.39 (5) CRU 0.01 0.15 (6) MSQ - - (7) SDS Unit - - Annexure-V Liquid Effluent Generation Sl Particulars Unit Quantity Remarks No.