MSU Extension IPM Bulletin, Fall 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

MOTHS and BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed Distributional Information Has Been J.D
MOTHS AND BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed distributional information has been J.D. Lafontaine published for only a few groups of Lepidoptera in western Biological Resources Program, Agriculture and Agri-food Canada. Scott (1986) gives good distribution maps for Canada butterflies in North America but these are generalized shade Central Experimental Farm Ottawa, Ontario K1A 0C6 maps that give no detail within the Montane Cordillera Ecozone. A series of memoirs on the Inchworms (family and Geometridae) of Canada by McGuffin (1967, 1972, 1977, 1981, 1987) and Bolte (1990) cover about 3/4 of the Canadian J.T. Troubridge fauna and include dot maps for most species. A long term project on the “Forest Lepidoptera of Canada” resulted in a Pacific Agri-Food Research Centre (Agassiz) four volume series on Lepidoptera that feed on trees in Agriculture and Agri-Food Canada Canada and these also give dot maps for most species Box 1000, Agassiz, B.C. V0M 1A0 (McGugan, 1958; Prentice, 1962, 1963, 1965). Dot maps for three groups of Cutworm Moths (Family Noctuidae): the subfamily Plusiinae (Lafontaine and Poole, 1991), the subfamilies Cuculliinae and Psaphidinae (Poole, 1995), and ABSTRACT the tribe Noctuini (subfamily Noctuinae) (Lafontaine, 1998) have also been published. Most fascicles in The Moths of The Montane Cordillera Ecozone of British Columbia America North of Mexico series (e.g. Ferguson, 1971-72, and southwestern Alberta supports a diverse fauna with over 1978; Franclemont, 1973; Hodges, 1971, 1986; Lafontaine, 2,000 species of butterflies and moths (Order Lepidoptera) 1987; Munroe, 1972-74, 1976; Neunzig, 1986, 1990, 1997) recorded to date. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Microlepidoptera.Hu Redigit: Fazekas Imre
Microlepidoptera.hu Redigit: Fazekas Imre 5 2012 Microlepidoptera.hu A magyar Microlepidoptera kutatások hírei Hungarian Microlepidoptera News A journal focussed on Hungarian Microlepidopterology Kiadó—Publisher: Regiograf Intézet – Regiograf Institute Szerkesztő – Editor: Fazekas Imre, e‐mail: [email protected] Társszerkesztők – Co‐editors: Pastorális Gábor, e‐mail: [email protected]; Szeőke Kálmán, e‐mail: [email protected] HU ISSN 2062–6738 Microlepidoptera.hu 5: 1–146. http://www.microlepidoptera.hu 2012.12.20. Tartalom – Contents Elterjedés, biológia, Magyarország – Distribution, biology, Hungary Buschmann F.: Kiegészítő adatok Magyarország Zygaenidae faunájához – Additional data Zygaenidae fauna of Hungary (Lepidoptera: Zygaenidae) ............................... 3–7 Buschmann F.: Két új Tineidae faj Magyarországról – Two new Tineidae from Hungary (Lepidoptera: Tineidae) ......................................................... 9–12 Buschmann F.: Új adatok az Asalebria geminella (Eversmann, 1844) magyarországi előfordulásához – New data Asalebria geminella (Eversmann, 1844) the occurrence of Hungary (Lepidoptera: Pyralidae, Phycitinae) .................................................................................................. 13–18 Fazekas I.: Adatok Magyarország Pterophoridae faunájának ismeretéhez (12.) Capperia, Gillmeria és Stenoptila fajok új adatai – Data to knowledge of Hungary Pterophoridae Fauna, No. 12. New occurrence of Capperia, Gillmeria and Stenoptilia species (Lepidoptera: Pterophoridae) ………………………. -

02 October 2015 Radebeul-Germany
©Societas Europaea Lepidopterologica; download unter http://www.soceurlep.eu/ und www.zobodat.at XIXth European Congress Welcome .............................................................................................................................................................. 3 of Lepidopterology Programme ....................................................................................................................................................... 5 27 September – 02 October 2015 Monday, 28 September 2015 ........................................................................................................ 5 Radebeul · Germany Tuesday, 29 September 2015 ....................................................................................................... 7 Wednesday, 30 September 2015 ................................................................................................ 9 Thursday, 1 October 2015 ............................................................................................................ 10 Friday, 2 October 2015 ................................................................................................................... 14 Honouring Niels Peder Kristensen ............................................................................................... 15 Abstracts .......................................................................................................................................................... 16 Oral presentations .......................................................................................................................... -

Moths of the Kingston Study Area
Moths of the Kingston Study Area Last updated 30 July 2015 by Mike Burrell This checklist contains the 783 species known to have occurred within the Kingston Study. Major data sources include KFN bioblitzes, an earlier version created by Gary Ure (2013) and the Queen’s University Biological Station list by Kit Muma (2008). For information about contributing your sightings or to download the latest version of this checklist, please visit: http://kingstonfieldnaturalists.org/moths/moths.html Contents Superfamily: Tineoidea .................................................................................................................................................... 5 Family: Tineidae ........................................................................................................................................................... 5 Subfamily: Tineinae .................................................................................................................................................. 5 Family: Psychidae ......................................................................................................................................................... 5 Subfamily: Psychinae ................................................................................................................................................ 5 Superfamily: Gracillarioidea ............................................................................................................................................. 5 Family: Gracillariidae ................................................................................................................................................... -

Desktop Biodiversity Report
Desktop Biodiversity Report Southwater Parish SxBRC/16/548 Prepared for Jennifer Nagy (Southwater Parish Council) 11th November 2016 ADDENDUM TO SxBRC/16/548 Sussex Protected Species Register Report It has been brought the Steering Group’s attention that the following evidence of Door Mice has been found within the Parish which is not mentioned in this report: 2012 Nut Hunt – PTES confirmed Dormouse chewed nuts found at TQ16405 27394 2018 PTES confirmed summer Dormouse nest found in hedgerow at TQ16414 27333 Sussex Biodiversity Record Centre desktop report regarding Southwater Parish 11th November 2016 Prepared for Jennifer Nagy Southwater Parish Council SxBRC/16/548 The following information was requested: Information Available Requested Format Designated Sites, Habitats & Ownership Maps Yes PDF Sussex Protected Species Register Yes Excel Sussex Bat Inventory Yes Excel Sussex Notable Bird Report Yes Excel UK BAP Species Inventory Yes Excel Sussex Rare Species Inventory Yes Excel Sussex Invasive Alien Species Yes Excel Full Species List Yes Excel Environmental Survey Directory No The following designations are within the search area: Local Wildlife Sites H08 ‐ Sparrow Copse H33 ‐ The Downs Link, Nutham Wood & Greatsteeds Farm Meadow H50 ‐ Courtland Wood H70 ‐ Southwater Country Park Complex Sites of Special Scientific Interest None Other Designations/Ownership Country Park Environmental Stewardship Agreement Important information regarding this report It must not be assumed that this report contains the definitive species information for the site concerned. The species data held by the Sussex Biodiversity Record Centre (SxBRC) is collated from the biological recording community in Sussex. However, there are many areas of Sussex where the records held are limited, either spatially or taxonomically. -
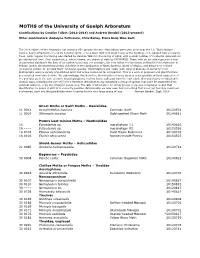
Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Earley, Fiona Reid, Mike Kent The 2018 edition of the Arboretum list contains 851 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent a first documented sighting if the date of an earlier record was not available. We now follow the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a group of species that can't be separated from external features, or by identifying to genus only. -

(Lepidoptera) Inhabiting the Funk Bottoms Wildlife Area, Wayne and Ashland Counties, Ohio1
Survey of the Moths (Lepidoptera) Inhabiting the Funk Bottoms Wildlife Area, Wayne and Ashland Counties, Ohio1 R. N. WILLIAMS, R. W. RINGS, M. S. ELLIS, AND D. S. FICKLE, Department of Entomology, Ohio Agricultural Research and Development Center, The Ohio State University, 1680 Madison Avenue, Wooster, OH 44691 ABSTRACT. In 1995, the Funk Bottoms Wildlife Area was the subject of an ongoing series of insect surveys intended to establish benchmark information on arthropod diversity of wetlands in northeast Ohio. This article concentrates on the moths which were collected at ultraviolet light traps within the Funk Bottoms Wildlife Area. A companion report will follow focusing on the Coleoptera along with several orders of aquatic insects. 3252 specimens were identified to 306 species in 19 families. These species are classified as follows: Abundant = 34; Locally Abundant = 1; Common = 257; Locally Common = 2; Uncommon = 10; Rare = 1; and Special Interest = 1. OHIO J. SCI. 97 (3): 34-39, 1997 INTRODUCTION Army Corps of Engineers. All the land behind the dam, The Funk Bottoms Wildlife Area was founded in 1991 below an elevation of 294 m, is under flood easement. with the initial purchase of land. The fact that this Wild- As a result, 3560 ha, including the Funk Bottoms Wildlife life Area is so new only magnifies the importance of Area, make up this wetland. There is relatively little area understanding the species existing there and what effect (around 80 ha) of permanently wet soils (marsh) within environmental changes might have upon them. This the easement area. However, hundreds to thousands of Wildlife Area currently consists of 467 ha which will be hectares may be inundated for periods of days to several expanded as funds and lands become available. -
University of Guelph ARBORETUM MOTHS All Species Found by CANDICE TALBOT (CT) Unless Otherwise Indicated
University of Guelph ARBORETUM MOTHS All species found by CANDICE TALBOT (CT) unless otherwise indicated. Other contributors: AB – Andrew Bendall AT – Andalyne Tofflemire CE – Chris Earley FR – Fiona Reid MK – Mike Kent Some notes about the records: Most moths were found at or near the J.C. Taylor Nature Centre, coming to building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by CT in 2012, with a small number of incidental observations pre- dating that time. Dates are written as 20140503 (e.g. May 3, 2014) and photographic records likely exist for all specific dates listed. Dates in brackets indicate that the moth was first recorded as present by CT before that date, but an image may not be available for the earlier sight record. Our list follows the order and groups of Beadle & Leckie (2012) Peterson Field Guide to Moths of Northeastern North America. Identifications are based on photographs using this guide as well as other published print and online resources for comparison. We acknowledge that, in some cases, definitive identification may not be possible without dissection and professional examination of the genitalia. Therefore some of our species identifications for difficult groups should be considered tentative. In other cases identification to genus is all that is possible, given the current knowledge of some groups or species complexes. Moss-Eaters - Family Micropterigidae 1. Goldcap Moss-eater Epimartyria auricrinella 20140530 AB Ghost Moths – Family Hepialidae 2. Lupulina Ghost Moth Korscheltellus lupulina 20120531 AB 3. Gold-spotted Ghost Moth Sthenopis auratus 20140621 Family Nepticulidae 4. -
Microlepidoptera.Hu 15 2019
Microlepidoptera.hu 15 2019 Aglossa caprealis (Hübner, [1809]) | Pyralidae Fotó / Photo: Csaniga Krisztina Redigit Fazekas Imre Pannon Intézet | Pannon Institute | Pécs | Hungary 2019 Microlepidoptera.hu 15: 1–41. | 02.12.2019 | HU ISSN 2062–6738 DOI: 10.24386/Microlep.2019.15.1 A folyóirat évente 1–3 füzetben jelenik meg. Taxonómiai, faunisztikai, állatföldrajzi, ökológiai és természetvédelmi tanulmányokat közöl Magyarországról és más földrajzi területekről. Az archivált publikációk az Országos Széchenyi Könyvtár Elektronikus Periodika Adatbázis és Archívumban (EPA) érhetők el: http://epa.oszk.hu/microlepidoptera valamint REAL J | EBSCO A folyóirat, nyomtatott formában, a szerkesztő címén megrendelhető. Hungarian Microlepidoptera News. A journal focused on Hungarian Microlepidopterology. Can be purchased in printed form and in CD. For single copies and further information contact the editor. Szerkesztő | Editor Fazekas Imre E-mail: [email protected] Web: www.microlepidoptera.hu A szerkesztő munkatársai | The editor’s assistants Ábrahám Levente (H-Kaposvár), Bálint Zsolt (H-Budapest), Barry Goater (GB-Eastleigh), Buschmann Ferenc (H-Jászberény), Gál Miklós (H-Komló), Nowinszky László (H- Szombathely), Puskás János (H-Szombathely), Pastorális Gábor (SK-Komárno), Szeőke Kálmán (H-Székesfehérvár), Tóth Sándor (H-Zirc) Kiadványterv, tördelés, tipográfia | Design, lay-out, typography: Fazekas Imre Kiadó | Publisher: Pannon Intézet | Pannon Institute | H-Pécs Nyomtatás | Print: Rotari Nyomdaipari Kft., H-Komló Megjelent | Published: -
Evaluation of Pathways for Exotic Plant Pest Movement Into and Within the Greater Caribbean Region
Evaluation of Pathways for Exotic Plant Pest Movement into and within the Greater Caribbean Region Caribbean Invasive Species Working Group (CISWG) and United States Department of Agriculture (USDA) Center for Plant Health Science and Technology (CPHST) Plant Epidemiology and Risk Analysis Laboratory (PERAL) EVALUATION OF PATHWAYS FOR EXOTIC PLANT PEST MOVEMENT INTO AND WITHIN THE GREATER CARIBBEAN REGION January 9, 2009 Revised August 27, 2009 Caribbean Invasive Species Working Group (CISWG) and Plant Epidemiology and Risk Analysis Laboratory (PERAL) Center for Plant Health Science and Technology (CPHST) United States Department of Agriculture (USDA) ______________________________________________________________________________ Authors: Dr. Heike Meissner (project lead) Andrea Lemay Christie Bertone Kimberly Schwartzburg Dr. Lisa Ferguson Leslie Newton ______________________________________________________________________________ Contact address for all correspondence: Dr. Heike Meissner United States Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine Center for Plant Health Science and Technology Plant Epidemiology and Risk Analysis Laboratory 1730 Varsity Drive, Suite 300 Raleigh, NC 27607, USA Phone: (919) 855-7538 E-mail: [email protected] ii Table of Contents Index of Figures and Tables ........................................................................................................... iv Abbreviations and Definitions .....................................................................................................