Dedolomitization and Other Diagenesis in the Backreef Setting of the Permian Reef Complex in Dark Canyon, New Mexico
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CONTROLS on DOLOMITIZATION of the UPPER ORDOVICIAN TRENTON LIMESTONE in SOUTH-CENTRAL KENTUCKY COLLIN JAMES GRAY Department Of
CONTROLS ON DOLOMITIZATION OF THE UPPER ORDOVICIAN TRENTON LIMESTONE IN SOUTH-CENTRAL KENTUCKY COLLIN JAMES GRAY Department of Geological Sciences APPROVED: Dr. Katherine Giles, Ph.D., Chair Dr. Richard Langford, Ph.D. Dr. Matthew Johnston, Ph.D. Charles Ambler, Ph.D. Dean of the Graduate School Copyright © by Collin James Gray 2015 Dedication I dedicate my thesis work to my family. My dedicated and loving parents, Michael and Deborah Gray have always supported me and provided words of encouragement during the struggles of my research. The support provided was second to none and I could not imagine reaching this point in my education without them. I also dedicate this thesis to my two brothers, Michael and Nathan Gray and my sister Nicole Gray. Without these role models I cannot imagine where my education would have ended. I will always appreciate the support provided by all three of you and consider you to be role models that I can always look up to. CONTROLS ON DOLOMITIZATION OF THE UPPER ORDOVICIAN TRENTON LIMESTONE IN SOUTH-CENTRAL KENTUCKY by COLLIN JAMES GRAY, B.S. GEOLOGY THESIS Presented to the Faculty of the Graduate School of The University of Texas at El Paso in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Department of Geological Sciences THE UNIVERSITY OF TEXAS AT EL PASO December 2015 Acknowledgements I wish to thank my committee whose time and expertise provided excellent input into my research. Dr. Katherine Giles, my M.S. supervisor provided guidance and provided endless suggestions and recommendations throughout my research while continuously motivating me to continue my education. -

Dolomite: Occurrence, Evolution and Economically Important Associations
Earth-Science Reviews 52Ž. 2000 1±81 www.elsevier.comrlocaterearscirev Dolomite: occurrence, evolution and economically important associations John Warren Department Petroleum Geosciences, UniÕersity Brunei Darussalam, Tungku Link, Bandar Seri Begawan, Brunei Received 14 October 1999; accepted 7 July 2000 Abstract Dolomite is not a simple mineral; it can form as a primary precipitate, a diagenetic replacement, or as a hydrothermalrmetamorphic phase, all that it requires is permeability, a mechanism that facilitates fluid flow, and a sufficient supply of magnesium. Dolomite can form in lakes, on or beneath the shallow seafloor, in zones of brine reflux, and in early to late burial settings. It may form from seawater, from continental waters, from the mixing of basinal brines, the mixing of hypersaline brine with seawater, or the mixing of seawater with meteoric water, or via the cooling of basinal brines. Bacterial metabolism may aid the process of precipitation in settings where sulfate-reducing species flourish and microbial action may control primary precipitation in some hypersaline anoxic lake settings. Dolomite is a metastable mineral, early formed crystals can be replaced by later more stable phases with such replacements repeated a number of times during burial and metamorphism. Each new phase is formed by the partial or complete dissolution of an earlier dolomite. This continual re-equilibration during burial detracts from the ability of trace elements to indicate depositional conditions and resets the oxygen isotope signature of the dolomite at progressively higher temperatures. Because subsurface dolomite evolves via dissolution and reprecipitation, a bed of dolomite can retain or create porosity and permeability to much greater burial depths and into higher temperature realms than a limestone counterpart. -

Igneous and Metamorphic Reservoirs | by E
UNICORNS IN THE GARDEN OF GOOD AND EVIL: Part 8 – Igneous and Metamorphic Reservoirs | By E. R. (Ross) Crain, P.Eng. Unicorns are beautiful, mythical beasts, much sought after by us mere mortals. The same is true for petrophysical models for unconventional reservoirs. This is the eighth in a series of review articles outlining the simple beauty of some practical methods for log analysis of the unusual. IGNEOUS AND METAMORPHIC BASICS Igneous and metamorphic rocks form reservoirs in many parts of the world. Reservoir quality varies and most benefit from natural fractures. Although some geologists believe the hydrocarbons in some Figure 1. Eyjafjallajokull volcano, Iceland, 2010. granite reservoirs comes from deep in the earth, the majority of these reservoirs can Igneous rocks are classified in several ways Parent Rock Metamorphic be shown to contain hydrocarbons that – by composition, texture, and method of Equivalent migrated from conventional sedimentary emplacement. Generally speaking, the Sandstone Quartzite sources. composition (mineral mixture) determines Limestone, Dolomite Marble the log response, the texture determines the As a generalization, metamorphic rocks are name used for the mineral mixture, and the Basalt Schist or Amphibolite rocks that have been exposed to high heat method of emplacement determines the Shale Slate and pressure. The main causes are: texture and internal porosity structure (if any). Granite Schist • Contact metamorphism – changes in the rock due to heat from a nearby magma Intrusive igneous rocks are formed inside the Rhyolite Schist source. earth. This type of igneous rock cools very Table 1. Metamorphic rock origins. • Regional metamorphism – changes slowly and is produced by magma from the caused by widespread elevated heat and interior of the earth. -

University Microfilms International 300 N
INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round blkek mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. For blurred pages, a good image of the page can be found in the adjacent frame. If copyrighted materials were deleted, a target note will appear listing the pages in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photographed, a definite method of “sectioning” the material has been followed. It is customary to begin filming at the upper left hand comer of a large sheet and to continue from left to right in equal sections with small overlaps. If necessary, sectioning is continued again—beginning below the first row and continuing on until complete. -
Ucementation
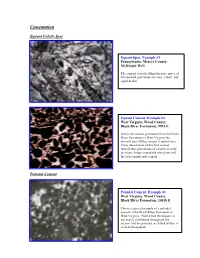
CementationU EquantU Calcite Spar PeloidalU Cement peloids. Equant Spar, Example #1 Pennsylvania, Mercer County, McKnight Well The cement crystals filling the pore space of this skeletal grainstone are very “clean” and equal in size. Equant Cement, Example #2 West Virginia, Wood County, Black River Formation, 9951 ft In this intraclastic grainstone from the Black River Formation in West Virginia the primary pore-filling cement is equant spar. Close observation of this thin section reveals two generations of cement an early prismatic fringe around the intraclasts and the later equant spar cement. PeloidalU Cement Peloidal Cement, Example #1 West Virginia, Wood County, Black River Formation, 10018 ft This is a typical example of a peloidal cement in the Black River Formation in West Virginia. Notice that the neospar is not evenly distributed throughout the section, but the peloidal or clotted texture is evident throughout. Peloidal Cement, Example #2 West Virginia, Wood County, Black River Formation, 10034 ft In this section the most distinct peloidal texture is evident in the lower left corner of the slide. In addition to the peloidal cement there are also wavy argillaceous laminations with associated dolomite crystals. Peloidal Cement, Example #3 Pennsylvania, Union Furnace outcrop The clotted texture of this mudstone shows the partial development of peloidal cement. Notice the somewhat rounded grains with sparry material in between. Further neomorphism will result in textures similar to those observed in the other peloidal cement slides. Peloidal Cement, Example #4 West Virginia, Wood County, Black River Formation, 10054 ft This peloidal cement was photographed under crossed polars. Notice the fuzzy grain boundaries between the peloids and the neospar DrusyU Spar Drusy Spar, Example #1 West Virginia, Wood County, Trenton Formation, 8495 ft Notice the different crystal sizes in this drusy calcite spar cement. -

Back Matter (PDF)
Index Page numbers in italics refer to Figures. Page numbers in bold refer to Tables. aggrading neomorphism 48 backscattered electron (BSE) imaging albite and albitization 9 Niger Delta Tertiary sands 249 authigenic 272–273, 277–278 Pannonian Basin study 407 cement 11 back-scattered electron microscopy (BSEM) 15 Algeria see Illizi Basin Arbuckle Group 283, 291–292 alkali elements, quartz cement study 378–379, barite 10, 11, 274–275 382–384, 384 baroque dolomite see under dolomite aluminium, quartz cement study 377–378, 378, 379–382, Barra Velha Formation 379, 384 Mg-rich clay mineral growth Amenas-Bourarhat Sud study area see Hirnantian chemical controls 36–38 glaciation sandstones diagenetic controls 39–41 analytical techniques, summary of 13–15 environment of formation 33–34 see also named methods sedimentological controls 38–39 anhydrite 10, 11, 13, 274–275 structural controls 34–36 Anschutz Ranch East Field (USA) 161 summary of behaviours 41–43 apatite cement 12 basin modelling 249–250 aragonite 4, 5 Niger Delta Tertiary 250–252 dissolution 47–48 Bassein Limestone in eodiagenesis 5 depositional environment 70 Arbuckle Group facies 71 geological setting 285–287 geological setting 68–70 methods of analysis 283–284 methods of analysis 72–73 results results BSEM 291–292 matrix porosity and stylolite density 74–76 fluid inclusion 292–300 pore system 73–74 paragenesis 287–288 results discussed early 288 diagenetic phases 76–77, 77 late 288–291 dissolution mechanism 79–83 Sr isotopes 306–309 porosity generation 77–79 stable isotopes 300–306 -

Microbial Alteration of Carbonate Archives
Microbial Alteration of Carbonate Archives Dissertation Zur Erlangung des Doktorgrades der Naturwissenschaften Dr. rer. nat. an der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrechts-Universität zu Kiel vorgelegt von Skadi Lange Kiel, 2017 1. Gutachterin: Prof. Dr. Tina Treude 2. Gutachter: Prof. Dr. Anton Eisenhauer Tag der Disputation: 12. September 2017 Hiermit erkläre ich, dass ich die vorliegende Doktorarbeit selbständig und ohne unerlaubte Hilfe erstellt habe. Weder diese noch eine ähnliche Arbeit wurde an einer anderen Abteilung oder Hochschule im Rahmen eines Prüfungsverfahrens vorgelegt, veröffentlicht oder zur Veröffentlichung vorgelegt. Ferner versichere ich, dass die Arbeit unter Einhaltung der Regeln guter wissenschaftlicher Praxis der Deutschen Forschungsgemeinschaft entstanden ist. Kiel, den Skadi Lange 54˚ 29.6' N 10° 15.1' E Table of contents Abstract 1 Zusammenfassung 5 Chapter 1 General Introduction 9 Chapter 2 Anaerobic microbial activity affects earliest diagenetic pathways of bivalve shells 25 Chapter 3 Effects of heterotrophic bacterial activity on coral aragonite during early diagenesis 63 Chapter 4 Effects of heterotrophic bacterial activity on foraminiferal tests 101 Chapter 5 Summary and final discussion 129 Acknowledgements 147 Abstract Marine biogenic carbonates are comprehensive archives of paleoenvironmental conditions. The main environmental variables that can be deduced from carbonate elemental- and isotopic composition are past sea-surface temperature, salinity and related ocean-circulation patterns, and -trace element concentration. These parameters are crucial to understand climate-linked paleo-ocean and atmospheric conditions, and to furthermore allow for interpolation to future environmental scenarios. However, the respective signals in carbonates can be biased by diagenetic alteration, and the abiotic processes involved in the diagenetic processes are being thoroughly investigated. -

An Example from the Mississippian Pekisko Formation, West-Central Alberta
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 2006 Petrologic and geochemical attributes of dolomite recrystallization: An example from the Mississippian Pekisko Formation, west-central Alberta. JoAnn Adam University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Adam, JoAnn, "Petrologic and geochemical attributes of dolomite recrystallization: An example from the Mississippian Pekisko Formation, west-central Alberta." (2006). Electronic Theses and Dissertations. 3075. https://scholar.uwindsor.ca/etd/3075 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Petrologic and geochemical attributes of dolomite recrystallization: an example from the Mississippian Pekisko Formation, west-central Alberta by JoAnn Adam A Thesis Submitted to the Faculty of Graduate Studies and Research through the Department of Earth Sciences in Partial Fulfillment of the Requirements for the Degree of Master of Science at the University of Windsor. -

The Relationship Between Dolomite Textures and Their Formation
Pet.Sci.(2014)11:39-51 39 DOI 10.1007/s12182-014-0316-7 The relationship between dolomite textures and their formation temperature: a case study from the Permian-Triassic of the Sichuan Basin and the Lower Paleozoic of the Tarim Basin Huang Sijing1, 2, Huang Keke1, 2 , Lü Jie1, 3 and Lan Yefang1, 3 1 State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Chengdu University of Technology, Chengdu, Sichuan 610059, China 2 Institute of Sedimentary Geology, Chengdu University of Technology, Chengdu, Sichuan 610059, China 3 College of Earth Sciences, Chengdu University of Technology, Chengdu, Sichuan 610059, China © China University of Petroleum (Beijing) and Springer-Verlag Berlin Heidelberg 2014 Abstract: Study of dolomite texture can contribute to understanding the process of dolomitization. This research reports textures and homogenization temperatures of dolomites from the Permian–Triassic strata in the Sichuan Basin and the Lower Paleozoic strata in the Tarim Basin, which provided insights into relationships between dolomite textures and their formation temperatures. Our results are summarized as follows: 1) dolomites with well-preserved texture indicate low dolomitization temperature. However, in certain diagenetic environments, the hydrothermal dolomitization may completely or partially preserve the original texture of dolomites. 2) The formation temperatures of non-planar dolomites are always higher than those of planar dolomites. 3) The formation temperatures of dolomite cements are generally higher than those of replacive dolomites. 4) Although the formation temperatures of saddle dolomite cements have a wide range, they show higher values than those of the planar subhedral to euhedral dolomite cements. Thus, saddle dolomites could generally be an indicator of high precipitation temperature. -

Metamorphic Rocks Metamorphism • Meta = Change, Greek • Morph
Metamorphic Rocks Metamorphism • Meta = change, Greek • Morph = form, Greek • Metamorphic rocks form from other rocks (protolith) by essentially solid-state changes in mineralogy and/or texture as a result of a change in chemical and/or physical environment. Metamorphism is characterized by: • phase changes - growth of new physically discrete, separable components (minerals), either with or without (isochemical) addition of new material; and/or • textural changes - recrystallization, alignment and/or grain size, usually as a result of unequal application of stress Agents of Metamorphism • Have been recrystallized as the result of – Temperature – Pressure • Confining pressure (compressive stress) • Directed pressure (differential stress), including shearing – Chemical Activity • the composition of pore fluids Pore Fluids • A fluid that occupies the empty spaces between particles • May come from – Partial melting of the rock – Groundwater Metamorphic Processes • Recrystallization • New Minerals-Index Minerals – Neomorphism: transformation of one mineral into a new one, depending upon its new environment. – Metasomatism: metamorphism coupled with the introduction of ions from an external source. (Ex: Water) • Mineral Orientation • Mineral Segregation Metamorphic Processes-How it is Done • Temperature changes – Below 200° not much happens – Metastability can occur – Migmatite can form from igneous rocks • Half igneous, half metamorphic Pressure Changes • Makes minerals pack themselves closer (mineral segregation) Chemically Active Fluids -

Digenesis, IV, Developments in Sedimentation
DEVELOPMENTS IN SEDIMENTOLOGY 51 Diagenesis, IV FURTHER TITLES IN THIS SERIES VOLUMES 1-11, 13-15, 17,21-25A, 27,28,31,32 and 39 are out of print 12 R.G.C. BATHURST CARBONATE SEDIMENTS AND THEIR DIAGENESIS 16 H.H. RlEKE Illand G.V. CHlLlNGARlAN COMPACTION OF ARGILLACEOUS SEDIMENTS 18A G.V. CHlLlNGARlAN and K.H. WOLF, Editors COMPACTION OF COARSE-GRAINED SEDIMENTS, I 188 G.V. CHlLlNGARlAN and K.H. WOLF, Editors COMPACTION OF COARSE-GRAINED SEDIMENTS, II 19 W. SCHARZACHER SEDIMENTATION MODELS AND QUANTITATIVE STRATIGRAPHY 20 M.R. WALTER, Editor STROMATOLITES 258 G. LARSEN and G.V. CHILINGAR, Editors DIAGENESIS IN SEDIMENTS AND SEDIMENTARY ROCKS 26 T. SUDO and S. SHIMODA, Editors CLAYS AND CLAY MINERALS OF JAPAN 29 P. TURNER CONTINENTAL RED BEDS 30 J.R.L. ALLEN SEDIMENTARY STRUCTURES 33 G.N. 6ATURlN PHOSPHORITES ON THE SEA FLOOR 34 J.J. FRIPIAT, Editor ADVANCED TECHNIQUES FOR CLAY MINERAL ANALYSIS 35 H. VAN OLPHEN and F.VENIALE, Editors INTERNATIONAL CLAY CONFERENCE 1981 36 A. IIJIMA, J.R. HElN and R. SEVER, Editors SILICEOUS DEPOSITS IN THE PACIFIC REGION 37 A. SlNGERand E. GALAN, Editors PALYGORSKITE-SEPIOLITE: OCCURRENCES, GENESIS AND USES 38 M.E. BROOKFIELD and T.S. AHLBRANDT, Editors EOLIAN SEDIMENTS AND PROCESSES 40 6. VELDE CLAY MINERALS-A PHYSICO-CHEMICAL EXPLANATION OF THEIR OCCURENCE 41 G.V. CHlLlNGARlAN and K.H. WOLF. Editors DIAGENESIS, I 42 L.J. DOYLE and H.H. ROBERTS, Editors CARBONATE-CLASTIC TRANSITIONS 43 G.V. CHlLlNGARlAN and K.H. WOLF, Editors DIAGENESIS, I1 44 C.E. WEAVER CLAYS, MUDS, AND SHALES 45 G.S. -

Quantifying Early Marine Diagenesis in Shallow-Water Carbonate Sediments
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta xxx (2018) xxx–xxx www.elsevier.com/locate/gca Quantifying early marine diagenesis in shallow-water carbonate sediments Anne-Sofie C. Ahm a,⇑, Christian J. Bjerrum b, Clara L. Bla¨ttler a, Peter K. Swart c, John A. Higgins a a Princeton University, Department of Geosciences, Guyot Hall, Princeton, NJ 08544, United States b University of Copenhagen, Nordic Center for Earth Evolution, Department of Geoscience and Natural Resource Management, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark c Department of Marine Geosciences, Rosenstiel School of Marine and Atmospheric Science, University of Miami, 4600 Rickenbacker Causeway, Miami, FL 33149, United States Received 4 August 2017; accepted in revised form 23 February 2018; available online xxxx Abstract Shallow-water carbonate sediments constitute one of the most abundant and widely used archives of Earth’s surface evo- lution. One of the main limitations of this archive is the susceptibility of the chemistry of carbonate sediments to post- depositional diagenesis. Here, we develop a numerical model of marine carbonate diagenesis that tracks the elemental and isotopic composition of calcium, magnesium, carbon, oxygen, and strontium, during dissolution of primary carbonates and re-precipitation of secondary carbonate minerals. The model is ground-truthed using measurements of geochemical prox- ies from sites on and adjacent to the Bahamas platform (Higgins et al., 2018) and authigenic carbonates in the organic-rich deep marine Monterey Formation (Bla¨ttler et al., 2015). Observations from these disparate sedimentological and diagenetic settings show broad covariation between bulk sediment calcium and magnesium isotopes that can be explained by varying the extent to which sediments undergo diagenesis in seawater-buffered or sediment-buffered conditions.