Quantifying Early Marine Diagenesis in Shallow-Water Carbonate Sediments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dust, Volcanic Ash, and the Evolution of the South Pacific Gyre Through the Cenozoic
Dust, volcanic ash, and the evolution of the South Pacific Gyre through the Cenozoic Dunlea, A. G., Murray, R. W., Sauvage, J., Spivack, A. J., Harris, R. N., & D'Hondt, S. (2015). Dust, volcanic ash, and the evolution of the South Pacific Gyre through the Cenozoic. Paleoceanography, 30(8), 1078-1099. doi:10.1002/2015PA002829 10.1002/2015PA002829 John Wiley & Sons, Inc. Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse PUBLICATIONS Paleoceanography RESEARCH ARTICLE Dust, volcanic ash, and the evolution of the South 10.1002/2015PA002829 Pacific Gyre through the Cenozoic Key Points: Ann G. Dunlea1, Richard W. Murray1, Justine Sauvage2, Arthur J. Spivack2, Robert N. Harris3, • Forty-seven element concentrations ’ 2 in 206 bulk sediment samples from and Steven D Hondt fi seven sites in the South Paci c 1 2 • Multivariate statistical models quantify Department of Earth and Environment, Boston University, Boston, Massachusetts, USA, Graduate School of 3 dust, ash, and other fluxes 100–0Ma Oceanography, University of Rhode Island, Narragansett, Rhode Island, USA, College of Earth, Ocean, and Atmospheric • Dust and ash records climate and Sciences, Oregon State University, Corvallis, Oregon, USA meridional shifts in atmospheric circulation Abstract We examine the 0–100 Ma paleoceanographic record retained in pelagic clay from the South Supporting Information: Pacific Gyre (SPG) by analyzing 47 major, trace, and rare earth elements in bulk sediment in 206 samples • Readme from seven sites drilled during Integrated Ocean Drilling Program Expedition 329. We use multivariate statistical • Figure S1 • Table S1 analyses (Q-mode factor analysis and multiple linear regression) of the geochemical data to construct a model • Table S2 of bulk pelagic clay composition and mass accumulation rates (MAR) of six end-members, (post-Archean • Table S3 average Australian shale, rhyolite, basalt, Fe-Mn-oxyhydroxides, apatite, and excess Si). -

September 24-25, 2004 FORWARD
Flow and Transport: Characterization and Modeling from Pore to Reservoir Scales C=0.2 C=0.4 Figure from Robert Glass Gaithersburg Marriott Washingtonian Center Gaithersburg, MD September 24-25, 2004 FORWARD “Flow and Transport: Characterization and Modeling from Pore to Reservoir Scales” is the eleventh in a series of Geosciences Research Program Symposia dating from 1995. These symposia are topically focused meetings for principal investigators in the program and provide opportunities for our investigators to give presentations to one another and to discuss their Office of Basic Energy Sciences’ supported research. In addition to the recognition the symposium gives to all of the investigators, we traditionally also recognize one outstanding contribution from a DOE Laboratory Project and one from a University Project. The outstanding contributions are selected by our session chairpersons. We are fortunate to have as guest session co-chairs Professor Roger Beckie from the University of British Columbia, Professor Ronald Falta from Clemson University, Professor Mario Ioannidis from the University of Waterloo, and Dr. Michael J. King from BP. They join our Principal Investigator co-chairs Professor Katherine McCall of the University of Nevada, Professor Amos Nur of Stanford University, Dr. Karsten Pruess of the Lawrence Berkeley National Laboratory, Dr. Wenlu Zhu of the Woods Hole Oceanographic Institution. For their efforts on behalf of the investigators I thank them all. We are looking forward to an outstanding series of presentations. Nicholas B. Woodward Geosciences Research Program Office of Basic Energy Sciences U.S. Department of Energy * * * * * Table of Contents Agenda…………………………………………………………………………………… 3 Abstracts (listed in chronological order) Session 1 (September 24, A.M.)………………….……………………………… 7 Session 2 (September 24, P.M.).………………………………………………… 16 Session 3 (September 25, A.M.)…………………............................................... -

CONTROLS on DOLOMITIZATION of the UPPER ORDOVICIAN TRENTON LIMESTONE in SOUTH-CENTRAL KENTUCKY COLLIN JAMES GRAY Department Of
CONTROLS ON DOLOMITIZATION OF THE UPPER ORDOVICIAN TRENTON LIMESTONE IN SOUTH-CENTRAL KENTUCKY COLLIN JAMES GRAY Department of Geological Sciences APPROVED: Dr. Katherine Giles, Ph.D., Chair Dr. Richard Langford, Ph.D. Dr. Matthew Johnston, Ph.D. Charles Ambler, Ph.D. Dean of the Graduate School Copyright © by Collin James Gray 2015 Dedication I dedicate my thesis work to my family. My dedicated and loving parents, Michael and Deborah Gray have always supported me and provided words of encouragement during the struggles of my research. The support provided was second to none and I could not imagine reaching this point in my education without them. I also dedicate this thesis to my two brothers, Michael and Nathan Gray and my sister Nicole Gray. Without these role models I cannot imagine where my education would have ended. I will always appreciate the support provided by all three of you and consider you to be role models that I can always look up to. CONTROLS ON DOLOMITIZATION OF THE UPPER ORDOVICIAN TRENTON LIMESTONE IN SOUTH-CENTRAL KENTUCKY by COLLIN JAMES GRAY, B.S. GEOLOGY THESIS Presented to the Faculty of the Graduate School of The University of Texas at El Paso in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Department of Geological Sciences THE UNIVERSITY OF TEXAS AT EL PASO December 2015 Acknowledgements I wish to thank my committee whose time and expertise provided excellent input into my research. Dr. Katherine Giles, my M.S. supervisor provided guidance and provided endless suggestions and recommendations throughout my research while continuously motivating me to continue my education. -

1. Cenozoic and Mesozoic Sediments from the Pigafetta Basin, Leg
Larson, R. L., Lancelot, Y., et al., 1992 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 129 1. CENOZOIC AND MESOZOIC SEDIMENTS FROM THE PIGAFETTA BASIN, LEG 129, SITES 800 AND 801: MINERALOGICAL AND GEOCHEMICAL TRENDS OF THE DEPOSITS OVERLYING THE OLDEST OCEANIC CRUST1 Anne Marie Karpoff2 ABSTRACT Sites 800 and 801 in the Pigafetta Basin allow the sedimentary history over the oldest remaining Pacific oceanic crust to be established. Six major deposition stages and events are defined by the main lithologic units from both sites. Mineralogical and chemical investigations were run on a large set of samples from these units. The data enable the evolution of the sediments and their depositional environments to be characterized in relation to the paleolatitudinal motion of the sites. The upper part of the basaltic crust at Site 801 displays a complex hydrothermal and alteration evolution expressed particularly by an ochre siliceous deposit comparable to that found in the Cyprus ophiolite. The oldest sedimentary cover at Site 801 was formed during the Callovian-Bathonian (stage 1) with red basal siliceous and metalliferous sediments similar to those found in supraophiolite sequences, and formed near an active ridge axis in an open ocean. Biosiliceous sedimentation prevailed throughout the Oxfordian to Campanian, with rare incursions of calcareous input during the middle Cretaceous (stages 2, 4, and 5). The biosiliceous sedimentation was drastically interrupted during the Aptian-Albian by thick volcaniclastic turbidite deposits (stage 3). The volcanogenic phases are pervasively altered and the successive secondary mineral parageneses (with smectites, celadonite, clinoptilolite, phillipsite, analcime, calcite, and quartz) define a "mineral stratigraphy" within these deposits. -

Dolomite: Occurrence, Evolution and Economically Important Associations
Earth-Science Reviews 52Ž. 2000 1±81 www.elsevier.comrlocaterearscirev Dolomite: occurrence, evolution and economically important associations John Warren Department Petroleum Geosciences, UniÕersity Brunei Darussalam, Tungku Link, Bandar Seri Begawan, Brunei Received 14 October 1999; accepted 7 July 2000 Abstract Dolomite is not a simple mineral; it can form as a primary precipitate, a diagenetic replacement, or as a hydrothermalrmetamorphic phase, all that it requires is permeability, a mechanism that facilitates fluid flow, and a sufficient supply of magnesium. Dolomite can form in lakes, on or beneath the shallow seafloor, in zones of brine reflux, and in early to late burial settings. It may form from seawater, from continental waters, from the mixing of basinal brines, the mixing of hypersaline brine with seawater, or the mixing of seawater with meteoric water, or via the cooling of basinal brines. Bacterial metabolism may aid the process of precipitation in settings where sulfate-reducing species flourish and microbial action may control primary precipitation in some hypersaline anoxic lake settings. Dolomite is a metastable mineral, early formed crystals can be replaced by later more stable phases with such replacements repeated a number of times during burial and metamorphism. Each new phase is formed by the partial or complete dissolution of an earlier dolomite. This continual re-equilibration during burial detracts from the ability of trace elements to indicate depositional conditions and resets the oxygen isotope signature of the dolomite at progressively higher temperatures. Because subsurface dolomite evolves via dissolution and reprecipitation, a bed of dolomite can retain or create porosity and permeability to much greater burial depths and into higher temperature realms than a limestone counterpart. -

Igneous and Metamorphic Reservoirs | by E
UNICORNS IN THE GARDEN OF GOOD AND EVIL: Part 8 – Igneous and Metamorphic Reservoirs | By E. R. (Ross) Crain, P.Eng. Unicorns are beautiful, mythical beasts, much sought after by us mere mortals. The same is true for petrophysical models for unconventional reservoirs. This is the eighth in a series of review articles outlining the simple beauty of some practical methods for log analysis of the unusual. IGNEOUS AND METAMORPHIC BASICS Igneous and metamorphic rocks form reservoirs in many parts of the world. Reservoir quality varies and most benefit from natural fractures. Although some geologists believe the hydrocarbons in some Figure 1. Eyjafjallajokull volcano, Iceland, 2010. granite reservoirs comes from deep in the earth, the majority of these reservoirs can Igneous rocks are classified in several ways Parent Rock Metamorphic be shown to contain hydrocarbons that – by composition, texture, and method of Equivalent migrated from conventional sedimentary emplacement. Generally speaking, the Sandstone Quartzite sources. composition (mineral mixture) determines Limestone, Dolomite Marble the log response, the texture determines the As a generalization, metamorphic rocks are name used for the mineral mixture, and the Basalt Schist or Amphibolite rocks that have been exposed to high heat method of emplacement determines the Shale Slate and pressure. The main causes are: texture and internal porosity structure (if any). Granite Schist • Contact metamorphism – changes in the rock due to heat from a nearby magma Intrusive igneous rocks are formed inside the Rhyolite Schist source. earth. This type of igneous rock cools very Table 1. Metamorphic rock origins. • Regional metamorphism – changes slowly and is produced by magma from the caused by widespread elevated heat and interior of the earth. -

University Microfilms International 300 N
INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round blkek mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. For blurred pages, a good image of the page can be found in the adjacent frame. If copyrighted materials were deleted, a target note will appear listing the pages in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photographed, a definite method of “sectioning” the material has been followed. It is customary to begin filming at the upper left hand comer of a large sheet and to continue from left to right in equal sections with small overlaps. If necessary, sectioning is continued again—beginning below the first row and continuing on until complete. -
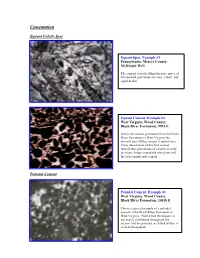
Ucementation
CementationU EquantU Calcite Spar PeloidalU Cement peloids. Equant Spar, Example #1 Pennsylvania, Mercer County, McKnight Well The cement crystals filling the pore space of this skeletal grainstone are very “clean” and equal in size. Equant Cement, Example #2 West Virginia, Wood County, Black River Formation, 9951 ft In this intraclastic grainstone from the Black River Formation in West Virginia the primary pore-filling cement is equant spar. Close observation of this thin section reveals two generations of cement an early prismatic fringe around the intraclasts and the later equant spar cement. PeloidalU Cement Peloidal Cement, Example #1 West Virginia, Wood County, Black River Formation, 10018 ft This is a typical example of a peloidal cement in the Black River Formation in West Virginia. Notice that the neospar is not evenly distributed throughout the section, but the peloidal or clotted texture is evident throughout. Peloidal Cement, Example #2 West Virginia, Wood County, Black River Formation, 10034 ft In this section the most distinct peloidal texture is evident in the lower left corner of the slide. In addition to the peloidal cement there are also wavy argillaceous laminations with associated dolomite crystals. Peloidal Cement, Example #3 Pennsylvania, Union Furnace outcrop The clotted texture of this mudstone shows the partial development of peloidal cement. Notice the somewhat rounded grains with sparry material in between. Further neomorphism will result in textures similar to those observed in the other peloidal cement slides. Peloidal Cement, Example #4 West Virginia, Wood County, Black River Formation, 10054 ft This peloidal cement was photographed under crossed polars. Notice the fuzzy grain boundaries between the peloids and the neospar DrusyU Spar Drusy Spar, Example #1 West Virginia, Wood County, Trenton Formation, 8495 ft Notice the different crystal sizes in this drusy calcite spar cement. -

Reservoir Quality Study of Siliciclastic and Carbonate
RESERVOIR QUALITY STUDY OF SILICICLASTIC AND CARBONATE ROCKS 3 days course in Oybin, Germany 12th - 14th of December 2018 Course Agenda 12th - 14th of December 2018 in Oybin, Germany The technical workshop (3 days oral & practical sessions) are aimed at giving specialists from the oil industry a detailed introduction to the study of siliciclastic & carbonate reservoirs. Workshops are structured in oral and practical sessions, with PPt presentations, didactic material/exercises and work at a polarising microscope. Examples of SEM and CL analyses are also shown to integrate the different methodologies utilized for diagenetic/reservoir quality studies. Examples from oil reservoirs from different basins in the world are also taken into consideration. RESERVOIR QUALITY STUDY OF SILICICLASTIC RESERVOIRS MODULE 1: Introduction. Siliciclastic rocks, classification of sedimentary rocks, type of petro-facies, sediment texture (sorting, grain size, grain shape, grain contacts, textural and mineralogical maturity), detrital components. Sandstone classification, ternary plots (Pettijohn, 1987), Optical properties of most important minerals of siliciclastic rocks under PPL and XPL (e.g. undulatory quartz) and their link with source areas (plutonic & metamorphic sources), depositional markers, chemical & mechanical stability of minerals Sandstone composition, provenance and tectonic settings: Data collection methods (Gazzi- Dickinson), litho-types vs. provenance (Dickinson plots), QFL of sedimentary rocks in different tectonic regimes and rock composition vs. porosity/burial depth. Other techniques for provenance studies (CL, Qemscan and geochemistry) will also be explained. Types of depositional environments, Provenance and reservoir quality. Examples from current East & West Africa reservoirs (e.g. Central Atlantic margins). Exercises. MODULE 2: Definition of matrix, pseudomatrix & authigenic components (main cements and replacement components): Compaction, silica cementation, carbonate cementation, feldspar authigenesis, clay minerals and zeolite authigenesis. -

Back Matter (PDF)
Index Page numbers in italics refer to Figures. Page numbers in bold refer to Tables. aggrading neomorphism 48 backscattered electron (BSE) imaging albite and albitization 9 Niger Delta Tertiary sands 249 authigenic 272–273, 277–278 Pannonian Basin study 407 cement 11 back-scattered electron microscopy (BSEM) 15 Algeria see Illizi Basin Arbuckle Group 283, 291–292 alkali elements, quartz cement study 378–379, barite 10, 11, 274–275 382–384, 384 baroque dolomite see under dolomite aluminium, quartz cement study 377–378, 378, 379–382, Barra Velha Formation 379, 384 Mg-rich clay mineral growth Amenas-Bourarhat Sud study area see Hirnantian chemical controls 36–38 glaciation sandstones diagenetic controls 39–41 analytical techniques, summary of 13–15 environment of formation 33–34 see also named methods sedimentological controls 38–39 anhydrite 10, 11, 13, 274–275 structural controls 34–36 Anschutz Ranch East Field (USA) 161 summary of behaviours 41–43 apatite cement 12 basin modelling 249–250 aragonite 4, 5 Niger Delta Tertiary 250–252 dissolution 47–48 Bassein Limestone in eodiagenesis 5 depositional environment 70 Arbuckle Group facies 71 geological setting 285–287 geological setting 68–70 methods of analysis 283–284 methods of analysis 72–73 results results BSEM 291–292 matrix porosity and stylolite density 74–76 fluid inclusion 292–300 pore system 73–74 paragenesis 287–288 results discussed early 288 diagenetic phases 76–77, 77 late 288–291 dissolution mechanism 79–83 Sr isotopes 306–309 porosity generation 77–79 stable isotopes 300–306 -

60° Geological and Petrophysical Parameters of a Deep Fractured Sandstone Formation As Applied to Geothermal Exploitation
60° Geological and petrophysical parameters of a deep fractured sandstone formation as applied to geothermal exploitation EPS-1 borehole, Soultz-sous-Forets, France *J.F. Vernoux, *A. Genter, *P. Razin, **C. Vinchon december 1995 Rapport du BRGM R 38622 *BRGM, Orléans **BRGM, Nord-Pas de Calais BRGM DIRECTION DE LA RECHERCHE Département Hydrologie, Géochimie et Transferts BP 6009 - 45060 ORLEANS CEDEX 2 - France - Tél.: (33) 38 64 34 34 Mots clés : Geothermal exploitation, Argillaceous sandstone, Sedimentological processes, Diagenesis, Fracture network, Reservoir, Porosity, Permeability, Soultz-sous-Forêts borehole, Rhinegraben, France En bibliographie, ce rapport sera cité de la façon suivante : VERNOUX J.F., GENTER A., RAZIN P., VINCHON C. (1995) - Geological and petrophysical parameters of a deep fractured sandstone formation as applied to geothermal exploitation, EPS-1 borehole, Soultz-sous- Forêts, France, Rapport BRGM R 38622, 70 p., 24 fig., 5 tabl., 3 append. © BRGM, 1995, ce document ne peut être reproduit en totalité ou en partie sans l'autorisation expresse du BRGM. Geological and petrophysical parameters of a deep fractured sandstone formation ABSTRACT In the framework of a European research project dealing with the improvement of the injectivity index of argillaceous sandstone, a basic study of a potential geothermal clastic aquifer was carried out from an exhaustive analysis of deep well data. A core from the base of Mesozoic cover rocks intersected in borehole EPS-1 (Soults-sous-Forêts, Rhine Graben, France) provided a continuous section of the Buntsandstein sandstones (1009-1417m), considered as a potential geothermal reservoir. More than 400 m length of continuous core sections were analyzed in details in terms of sedimentological features, diagenetic evolution, pre-existing fracturation and petrophysical properties in order to define the best potential reservoirs within this deep clastic formation. -

UNDER the ROCK SUNY Geneseo’S GSCI Annual Newsletter SUMMER 2018
UNDER THE ROCK SUNY Geneseo’s GSCI Annual Newsletter SUMMER 2018 Greetings from the chair: Happy end of summer to all of our Geneseo GSCI friends and family. My first year as chair Fall Brook waterfall, Geneseo has been a good one. I find I am very privileged to lead such a strong department On a department level, we have started to dedicate some that continues to inspire and nurture young intentional focus to making sure we are safe when we take geologists as well as make an impact on our students on field trips. To help us learn more about best understanding of the Earth and all its facets. practices regarding safety, we invited Kurt Burmeister (University of the Pacific) to come and present a “field Mineralogy and Petrology continued to serve large numbers safety course” to our department. It was co-sponsored by over the ’17-18 academic year. Mineralogy had a whopping 48 the Provost’s office and our department (thanks to all for students! They brought a zeal and curiosity that made for some your support). This training was attended by a student, all great term paper reading. It looks like this upcoming year will of the GSCI faculty and staff, and faculty from the Bio and be slightly smaller, but that will allow students to have easier Geography departments. It was a transformational event access to instruments such as the X-ray diffractometer. made us think more proactively about field safety, and we have already started to implement changes to our field In the fall, I was delighted to attend the national GSA meeting trips.