Modeling Bacteria-Phage Interactions and Its Implications for Phage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Islamic Geometric Patterns Jay Bonner
Islamic Geometric Patterns Jay Bonner Islamic Geometric Patterns Their Historical Development and Traditional Methods of Construction with a chapter on the use of computer algorithms to generate Islamic geometric patterns by Craig Kaplan Jay Bonner Bonner Design Consultancy Santa Fe, New Mexico, USA With contributions by Craig Kaplan University of Waterloo Waterloo, Ontario, Canada ISBN 978-1-4419-0216-0 ISBN 978-1-4419-0217-7 (eBook) DOI 10.1007/978-1-4419-0217-7 Library of Congress Control Number: 2017936979 # Jay Bonner 2017 Chapter 4 is published with kind permission of # Craig Kaplan 2017. All Rights Reserved. This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -

The Eagle 1939 (Easter)
96 THE EAGLE . THE EAGLE � I.QttQacQt 1.Q1.1.Q1 l.Q1c1tcQtcbtf.(/hf.Q1 cbt to't c.()'t '" cQt� I.Q1 eb't""" � COLLEGE AWARDS .,pt.,pt &Q'I.,pt tbtlbtc.QJcg.tQt No. 223 VOL. LI June 1939 � l.Q1 cQt cQt following awards were made on the results of the &Q'Ic.()'Ic.()'Ic.()'I&Q'I�cQtc.QJl.Q1f.(/hcQt l.Q1f.(/hcQtf.(/hl.Q1l.Q1c.()'tcQt c.()'tf.(/h l.Q1c.QJtQt�"' � THE Annual Entrance Scholarships Examination, December 1938: Major Scholarships: Read, A. H., Marlborough College, for Mathematics (Baylis Scholar- ship). Goldie, A. W., Wolverhampton Grammar School, for Mathematics. Charlesworth, G. R, Penis tone Grammar School, for Mathematics. Brough, J., Edinburgh University, for Classics. Howorth, R H., Manchester Grammar School, fo r Classics (Patchett Scholarship ). THE COMMEMORATION SERMON Freeman, E. J., King Edward VI School, Birmingham, for Classics. Crook, J. A., Dulwich College, for Classics. By THE MASTER, Sunday, 7 May 1939 Hereward, H. G., King Edward VI School, Birmingham, for Natural Sciences. T from the will of the Lady Robinson, R E., Battersea Grammar School, for History. me begin with a sentence Lapworth, H. J., King Edward VI School, Birmingham, for Modern Margaret: Languages. I "Be it remembered that it was also the last will of the said Minor Scholarships: princess to dissolve the hospital of Saint John in Cambridge Jones, R P. N., Manchester Grammar School, for Mathematics. persons, Bell, W. R G., Bradford Grammar School, for Mathematics. and to alter and to found thereof a College of secular Ferguson, J" Bishop's Stortford College, for Classics. -

The Roles of Moron Genes in the Escherichia Coli Enterobacteria Phage Phi-80
THE ROLES OF MORON GENES IN THE ESCHERICHIA COLI ENTEROBACTERIA PHAGE PHI-80 Yury V. Ivanov A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY December 2012 Committee: Ray A. Larsen, Advisor Craig L. Zirbel Graduate Faculty Representative Vipa Phuntumart Scott O. Rogers George S. Bullerjahn © 2012 Yury Ivanov All Rights Reserved iii ABSTRACT Ray Larsen, Advisor The TonB system couples cytoplasmic membrane-derived proton motive force energy to drive ferric siderophore transport across the outer membrane of Gram-negative bacteria. While much effort has focused on this process, how energy is harnessed to provide for transport of ligands remains unknown. Several bacterial viruses (“phage”) are known to require the TonB system to irreversibly adsorb (i.e., establish infection) in the model organism Escherichia coli. One such phage is φ80, a “cousin” of the model temperate phage λ. Determining how φ80 is using the TonB system for infection should provide novel insights to the mechanisms of TonB-dependent processes. It had long been known that recombination between λ and φ80 results in a λ-like phage for whom TonB is now required; and this recombination involved the λ J gene, which encodes the tail-spike protein required for irreversible adsorption of λ to E. coli. Thus, we suspected that a φ80 homologue of the λ J gene product was responsible for the TonB dependence of φ80. While φ80 has long served as a tool for assaying TonB activity, it has not received the scrutiny afforded λ. -
Buggy • A12 Tunes at Concert • B6 SCITECH SPORTS PILLBOX
Researchers study neuron SDC A team defends title, Matt and Kim present new response to morphine • A5 wins Buggy • A12 tunes at concert • B6 SCITECH SPORTS PILLBOX thetartan.org @thetartan April 20, 2015 Volume 109, Issue 24 Carnegie Mellon’s student newspaper since 1906 DEBORAH CHU firmly. Junior Staffwriter Casalegno described this occasion as “momentous,” This year’s Midway Open- drawing parallels between ing Ceremony for Spring Car- the all-encompassing essence nival paid a bittersweet fare- of Spring Carnival and the in- well to its longstanding place terdisciplinary nature of the on the Morewood Gardens future Tepper Quadrangle. parking lot. It touched on the The Quadrangle is scheduled new Tepper Quadrangle that to be built on the Morewood would take its place, and re- gardens parking lot, making minded us of the philanthro- this occasion also a hand-off. py aspect of Carnival. True “[The Tepper Quadrangle] to Carnegie Mellon tradition, is a new chapter in our cam- the air echoed with the sound pus history that will bring to- of bagpipes as Carnival offi- gether the whole of our cam- cially kicked off. pus … where collaboration People huddled inside will reign,” Casalegno said. the large Alumni Association “It will be a place where inno- Tent on the Morewood Gar- vation will thrive.” dens parking lot while rain As for Midway’s new home drizzled outside. Dean of Stu- on the CFA lawn, Casalegno dent Affairs Gina Casalegno points out that it would be joked that despite last year’s closer to other Carnival ac- announcement that Midway tivities such as Sweepstakes, would not take place at the more commonly known as Morewood Gardens park- Buggy. -

Exploitation Du Potentiel Des Bactériophages Dans Le Traitement Des Surfaces En Contact Avec L’Eau, Contaminées Par Un Biofilm De P
Exploitation du potentiel des bactériophages dans le traitement des surfaces en contact avec l’eau, contaminées par un biofilm de P. aeruginosa Vanessa Magin To cite this version: Vanessa Magin. Exploitation du potentiel des bactériophages dans le traitement des surfaces en contact avec l’eau, contaminées par un biofilm de P. aeruginosa. Ingénierie de l’environnement. Ecole nationale supérieure Mines-Télécom Atlantique, 2019. Français. NNT : 2019IMTA0146. tel-02391184 HAL Id: tel-02391184 https://tel.archives-ouvertes.fr/tel-02391184 Submitted on 3 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. • THESE DE DOCTORAT DE L’ÉCOLE NATIONALE SUPERIEURE MINES-TELECOM ATLANTIQUE BRETAGNE PAYS DE LA LOIRE - IMT ATLANTIQUE COMUE UNIVERSITE BRETAGNE LOIRE ECOLE DOCTORALE N° 602 Sciences pour l'Ingénieur Spécialité : Génie des Procédés et Bioprocédés Par Vanessa MAGIN Par Vanessa MAGIN Exploration du potentiel des bactériophages dans le traitement des surfaces en contact avec l’eau, contaminées par un biofilm de P.aeruginosa Thèse présentée et soutenue -

The History of Virology – the Scientific Study of Viruses and Therefore the Infections- Research Analysis of Virology and Retr
Current research in Virology & Retrovirology 2021, Vol.2, Issue 3 Short Communication The history of virology – the scientific study of viruses and therefore the infections- Research Analysis of Virology and Retrovirology Kwanighee Yonsei University, South Korea cholerae. Bacteriophages were heralded as a possible treatment for Copyright: 2021 Kwanighee . This is an open-access article distributed diseases like typhoid and cholera, but their promise was forgotten under the terms of the Creative Commons Attribution License, which with the event of penicillin. Since the early 1970s, bacteria have permits unrestricted use, distribution, and reproduction in any medium, continued to develop resistance to antibiotics such as penicillin, provided the original author and source are credited. and this has led to a renewed interest in the use of bacteriophages to treat serious infections. Early research 1920–1940, D'Herelle Abstract travelled widely to market the utilization of bacteriophages within the treatment of bacterial infections. In 1928, he became professor The history of virology – the scientific study of viruses and therefore of biology at Yale and founded several research institutes. He was the infections they cause – began within the closing years of the convinced that bacteriophages were viruses despite opposition 19th century. Although Pasteur and Jenner developed the primary from established bacteriologists such as the Nobel Prize winner vaccines to guard against viral infections, they didn't know that Jules Bordet (1870–1961). Bordet argued that bacteriophages viruses existed. The first evidence of the existence of viruses came from experiments with filters that had pores sufficiently small to were not viruses but just enzymes released from "lysogenic" retain bacteria. -

Samuel Vijaya Bhaskar Poldas, Geschichte Der Homöopathie In
Mitterweger & Partner GmbH #42674 MVS / Herr Rieder 3. Umbruch gd 29.06.2010 Poldas, Die Geschichte der Homöopathie in Indien Quellen und Studien zur Homöopathiegeschichte Herausgegeben vom Institut für Geschichte der Medizin der Robert Bosch Stiftung Leiter: Prof. Dr. phil. Robert Jütte Band 13 Die Drucklegung erfolgte mit finanzieller Unterstützung der Robert Bosch Stiftung GmbH, Stuttgart Mitterweger & Partner GmbH #42674 MVS / Herr Rieder 3. Umbruch gd 29.06.2010 Poldas, Die Geschichte der Homöopathie in Indien Geschichte der Homöopathie in Indien von ihrer Einführung bis zur ersten offiziellen Anerkennung 1937 Samuel Vijaya Bhaskar Poldas 26 Tabellen Karl F. Haug Verlag · Stuttgart Mitterweger & Partner GmbH #42674 MVS / Herr Rieder 3. Umbruch gd 29.06.2010 Poldas, Die Geschichte der Homöopathie in Indien Bibliografische Information Wichtiger Hinweis: Wie jede Wissenschaft ist die Medizin der Deutschen Nationalbibliothek ständigen Entwicklungen unterworfen. Forschung und klinische Erfahrung erweitern unsere Erkenntnisse, ins- Die Deutsche Nationalbibliothek verzeichnet diese besondere was Behandlung und medikamentöse Therapie Publikation in der Deutschen Nationalbibliografie; anbelangt. Soweit in diesem Werk eine Dosierung oder detaillierte bibliografische Daten sind im Internet eine Applikation erwähnt wird, darf der Leser zwar darauf über http://dnb.d-nb.de abrufbar. vertrauen, dass Autoren, Herausgeber und Verlag große Sorgfalt darauf verwandt haben, dass diese Angabe dem Wissensstand bei Fertigstellung des Werkes entspricht. Für Angaben über Dosierungsanweisungen und Appli- kationsformen kann vom Verlag jedoch keine Gewähr übernommen werden. Jeder Benutzer ist angehalten, durch sorgfältige Prüfung der Beipackzettel der verwen- deten Präparate und gegebenenfalls nach Konsultation eines Spezialisten festzustellen, ob die dort gegebene Empfehlung für Dosierungen oder die Beachtung von Kontraindikationen gegenüber der Angabe in diesem Buch abweicht. -

Museu Regional De Arte E Arqueologia Islâmica
Instituto Superior Manuel Teixeira Gomes MUSEU REGIONAL DE ARTE E ARQUEOLOGIA ISLÂMICA Dissertação conducente à obtenção do Grau de Mestre em Arquitetura | Mestrado Integrado em Arquitetura | Grupo Lusófona Discente: Estela Maria do Carmo Samuel; nº. 21100164 Orientador: Professor Doutor Mostafa Zekri Portimão | 2017 Instituto Superior Manuel Teixeira Gomes MUSEU REGIONAL DE ARTE E ARQUEOLOGIA ISLÂMICA Dissertação conducente à obtenção do Grau de Mestre em Arquitetura | Mestrado Integrado em Arquitetura | Grupo Lusófona Discente: Estela Maria do Carmo Samuel; nº. 21100164 Orientador: Professor Doutor Mostafa Zekri Portimão | 2017 ESTELA MARIA DO CARMO SAMUEL MUSEU REGIONAL DE ARTE E ARQUEOLOGIA ISLÂMICA. Dissertação defendida em provas públicas no Instituto Superior Manuel Teixeira Gomes, no dia 27/03/2017 perante o júri nomeado pelo Despacho de Nomeação nº. 02/2017, com a seguinte composição: Presidente: Prof.ª Doutora Ana Cristina Santos Bordalo (Professora Auxiliar, ISMAT) Arguente: Prof. Doutor Luís Filipe Pires Conceição (Professor Catedrático Convidado, ISMAT) Orientador: Prof. Doutor Mostafa Zekri (Professor Associado, ISMAT) Instituto Superior Manuel Teixeira Gomes Portimão 2017 Museu Regional de Arte e Arqueologia Islâmica Agradecimentos Ao meu orientador Professor Doutor Mostafa Zekri em especial pela sua dedicação e mestria na orientação com o meu trabalho, em pesquisas efetuadas em livros sobre a arte e arquitetura Islâmica; e outros que foram essenciais para a elaboração desta dissertação com sucesso. Um agradecimento aos meus professores, que ajudaram na minha formação académica, de uma forma magnífica, na minha cultura e desenvolvimento artístico, aos meus colegas de turma, que me acompanharam desde a semana académica, em especial o Carlos; Sandra Aires; Lígia Agostinho; Rita André; Savannah Salgueiro; João Rego e o Carlos Nascimento, aos funcionários do ISMAT, que foram sempre de uma grande simpatia e profissionalismo Um agradecimento muito especial ao Exmo. -

POLITECNICO DI TORINO Repository ISTITUZIONALE
POLITECNICO DI TORINO Repository ISTITUZIONALE La progettazione parametrica come strumento di analisi: dai pattern algoritmici decorativi ai pattern “performanti”, esempi nei Beni Culturali Original La progettazione parametrica come strumento di analisi: dai pattern algoritmici decorativi ai pattern “performanti”, esempi nei Beni Culturali / Fassino, Mauro. - (2012). Availability: This version is available at: 11583/2497523 since: Publisher: Politecnico di Torino Published DOI:10.6092/polito/porto/2497523 Terms of use: openAccess This article is made available under terms and conditions as specified in the corresponding bibliographic description in the repository Publisher copyright (Article begins on next page) 04 August 2020 4 Pattern nella geometria euclidea «As David Hilbert pointed out, the logical structure of geometry should remain equally valid if the words “point, line, plane” are replaced by “beer-mug, chair, table” ». «Come sottolineò David Hilbert, la struttura logica della geometria dovrebbe rimanere ugualmente valida qualora le parole “punto, linea, piano” fossero sostituite con "boccale di birra, sedia, tavolo”». IAN STEWART , Geometry in Victorian England , «New Scientist», n. 1657, 1989, p. 60. Sicuramente i pattern più diffusi sono quelli destinati ad essere riprodotti nello spazio euclideo. Tutte le tassellazioni e i pattern finora descritti sono infatti concepiti per essere disegnati all’interno del piano cartesiano, in un contesto che ha trovato, oggi come in passato, le maggiori applicazioni nell’ambito decorativo e architettonico. Tuttavia il loro uso sembra per il momento limitato alla ricerca matematica e al campo artistico, dove ha prodotto risultati di alto livello estetico; risulta, invece, più complesso impiegarli per ottenere performance strutturali o decorative in architettura, poiché le accortezze e le perizie tecniche che richiedono ne rendono difficoltoso l’impiego. -

The Virus in the Rivers: Histories and Antibiotic Afterlives of the Bacteriophage at the Sangam in Allahabad
The virus in the rivers: histories and antibiotic afterlives of the bacteriophage at the sangam in Allahabad The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Kochhar, Rijul. "The virus in the rivers: histories and antibiotic afterlives of the bacteriophage at the sangam in Allahabad." Notes and Records: The Royal Society Journal of the History of Science 74, 4 (July 2020): 625–651 © 2020 The Author(s) As Published http://dx.doi.org/10.1098/rsnr.2020.0019 Publisher Royal Society Publishing Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/128370 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Notes Rec. doi:10.1098/rsnr.2020.0019 Published online THE VIRUS IN THE RIVERS: HISTORIES AND ANTIBIOTIC AFTERLIVES OF THE BACTERIOPHAGE AT THE SANGAM IN ALLAHABAD by RIJUL KOCHHAR* Massachusetts Institute of Technology, Doctoral Program in History, Anthropology, Science, Technology, and Society (HASTS), 77 Massachusetts Avenue, E51-163, Cambridge, MA 02139, USA The confluence (sangam) of India’s two major rivers, the Ganges and the Yamuna, is located in the city of Allahabad. Ritualistic dips in these river waters are revered for their believed curative power against infections, and salvation from the karmic cycles of birth and rebirth. The sacred and geographic propensities of the rivers have mythic valences in Hinduism and other religious traditions. Yet the connection of these river waters with curativeness also has a base in historical microbiology: near here, the British bacteriologist Ernest Hanbury Hankin, in 1896, first described the ‘bactericidal action of the waters of the Jamuna and Ganges rivers on Cholera microbes’, predating the discovery of bacterial viruses (now known as bacteriophages) by at least two decades. -

Obituary Notices Prof
No. 3626, APRIL 29, 1939 NATURE 711 The use of deuterium as an indicator was dis respiration, in which the heaviness of the expired cussed by Prof. H. S. Raper and Dr. W. E. van carbon dioxide was determined. Heyningen. The former referred to the work of Radioactive sodium was dealt with by Dr. Cavanagh and Raper, in which fats labelled with B. G. Maegraith, who mentioned experiments in deuterium were fed to animals and a study made which active sodium chloride had been injected of the rate of formation of the deuterium labelled into rabbits, and the distribution of the active lipins in the liver and kidney; the latter gave an sodium investigated. He suggested that this might account of the work of Schoenheimer and Ritten be used to estimate the extracellular fluid content berg et al. on deuterium as an indicator in the of the rabbit. study of intermediary metabolism, and referred Dr. W. D. Armstrong described experiments especially to the uses and limitations of the dealing with the exchange of phosphorus of the method. enamel of teeth and the blood using radioactive The use of heavy oxygen as an indicator was phosphorus. A very slow exchange was noticed, discussed by Dr. J. N. E. Day. He referred to indicating, not the formation of new molecules, the work of Aten and Hevesy, who examined the but exchange of phosphate between enamel and possibility of exchange of oxygen in sulphate, with blood. Mr. C. H. Collie referred to work of Collie other oxygen atoms present in the body, by and Morgan showing that radioactive sulphur can injecting heavy sodium sulphate into rabbits, and be used as an indicator. -
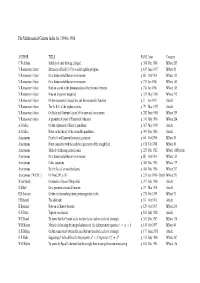
The Mathematical Gazette Index for 1894 to 1908
The Mathematical Gazette Index for 1894 to 1908 AUTHOR TITLE PAGE Issue Category C.W.Adams Stability of cube floating in liquid p. 388 Dec 1908 MNote 285 V.Ramaswami Aiyar Extension of Euclid I.47 to n-sided regular polygons p. 109 June 1897 MNote 41 V.Ramaswami Aiyar On a fundamental theorem in inversion p. 88 Oct 1904 MNote 153 V.Ramaswami Aiyar On a fundamental theorem in inversion p. 275 Jan 1906 MNote 183 V.Ramaswami Aiyar Note on a point in the demonstration of the binomial theorem p. 276 Jan 1906 MNote 185 V.Ramaswami Aiyar Note on the power inequality p. 321 May 1906 MNote 192 V.Ramaswami Aiyar On the exponential inequalities and the exponential function p. 8 Jan 1907 Article V.Ramaswami Aiyar The A, B, C of the higher analysis p. 79 May 1907 Article V.Ramaswami Aiyar On Stolz and Gmeiner’s proof of the sine and cosine series p. 282 June 1908 MNote 259 V.Ramaswami Aiyar A geometrical proof of Feuerbach’s theorem p. 310 July 1908 MNote 264 A.O.Allen On the adjustment of Kater’s pendulum p. 307 May 1906 Article A.O.Allen Notes on the theory of the reversible pendulum p. 394 Dec 1906 Article Anonymous Proof of a well-known theorem in geometry p. 64 Oct 1896 MNote 30 Anonymous Notes connected with the analytical geometry of the straight line p. 158 Feb 1898 MNote 49 Anonymous Method of reducing central conics p. 225 Dec 1902 MNote 110B (note) Anonymous On a fundamental theorem in inversion p.