Conversion of Alcohols Into Amines by Borrowing Hydrogen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrogenation of Styrene Oxide to 2-Phenylethanol Over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method
Hindawi Publishing Corporation International Journal of Chemical Engineering Volume 2014, Article ID 406939, 6 pages http://dx.doi.org/10.1155/2014/406939 Research Article Hydrogenation of Styrene Oxide to 2-Phenylethanol over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method Sunil K. Kanojiya,1 G. Shukla,1 S. Sharma,1 R. Dwivedi,2 P. Sharma,2 R. Prasad,2 M. Satalkar,3 and S. N. Kane3 1 IPCA Laboratories Ltd., Indore 452003, India 2 School of Chemical Sciences, DA University, Indore 452001, India 3 Magnetic Materials Laboratory, School of Physics, DA University, Indore 452001, India Correspondence should be addressed to R. Prasad; [email protected] Received 14 February 2014; Accepted 20 May 2014; Published 17 July 2014 Academic Editor: Moshe Sheintuch Copyright © 2014 Sunil K. Kanojiya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Nanocrystalline nickel prepared by glycol reduction method and characterized by XRD and magnetic measurements has been used as a catalyst for hydrogenation of styrene oxide to 2-phenylethanol. Effect of process variables such as particle size of the catalyst, temperature, and pressure have been optimized to achieve a maximum conversion of 98% of styrene oxide with 99% selectivity towards 2-phenylethanol. The structure of the transition state has been computed employing density functional theory −1 and using Gaussian 09 suite. The enthalpy of reaction (Δ) and activation energy ()arecalculatedtobe85.3kcal⋅mol and −1 123.03 kcal⋅mol , respectively. A tentative mechanism for the reaction is proposed according to which atomized hydrogen and styrene oxide react together over the catalyst surface to produce 2-phenylethanol. -

Green Chemistry : Greener Alternatives to Synthetic Organic Transformations
Green Chemistry Greener Alternatives to Synthetic Organic Transformations V.K. Ahluwalia Alpha Science International Ltd. Oxford, U.K. Contents Preface v Parti 1. Introduction 1.1-1.10 1.1 Principles of Green Chemistry 1.1 1.2 How to Plan a Green Synthesis 1.2 Part II Green Alternatives to Synthesis Organic Transformations 2. Aqueous Phase Transformations 2.1-2.48 2.1 p-Acetylaminophenol (Tylenol) 2.1 2.2 3-Aminopyridine 2.2 2.2a Anthranilic Acid 2.3 2.3 Benzilic Acid 2.3 2.4 Benzoin 2.5 2.5 Benzotriazole 2.6 2.6 2-Benzoyl-3,5-dimethylbenzofuran 2.7 2.7 n-Butyl Bromide 2.8 2.8 Tert.Butylchloride 2.8 2.9 Chalcone (Benzalacetophenone) 2.9 2.10 Cycloheptanone 2.10 2.11 2,3-Dihydroxy Anisole (Pyrigallol Monomethyl-ether) 2.12 2.12 2,4-Dihydroxybenzoic acid (P-resorcylic Acid) 2.13 2.13 3,4-Dimethoxyphenol 2.14 2.14 2,3-Dimethyl-l-phenylpyrazol-5-one 2.15 2.15 3, 5-Dimethylpyrazole 2.16 2.16 5,5-Diphenylhydantoin 2.17 2.17 Endo-cis-1,4-endoxo-A5-cyclohexene-2,3-dicarboxylic Acid 2.18 2.18 p-Ethoxyacetanilide (Phenacetin) 2.19 2.19 6-Ethoxycarbonyl-3,5-diphenyI-2-cyclohexenone 2.20 2.20 HeteroDiels-AlderAdduct 2.22 2.21 Hippuric Acid (Benzoyl Glycine) 2.22 Viii Contents 2.22 Hydantion 2.23 2.25 3-Hydroxy-3-phenyl-2-methylene Proponamide 2.24 2.24 Iodoform 2.25 2.25 Inodole 2.26 2.26 3-(p-Methoxyphenyl)-2H-1,4-Benzoxazine 2.27 2.27 3-Methylcyclopent-2-enone 2.27 2.28 2-(2'-Methlindol-3yl)-1,4-benzoquinone 2.28 2.29 2-methyl-2-(3-oxobutyl)-1,3-cyclopentanedione 2.29 2.30 P-Naphthyl Acetate 2.30 2.31 (5-Naphthyl Methyl Ether (Nerolin) 2.31 2.32 -

Inhibition of Growth, Synthesis, and Permeability in Neurospora Crassa by Phenethyl Alcohol
JOURNAL OF BACTERIOLOGY, JUly, 1965 Vol. 90, No. I Copyright © 1965 American Society for Microbiology Printed in U.S.A. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol GABRIEL LESTER Department of Biology, Reed College, Portland, Oregon Received for publication 22 January 1965 ABSTRACT LESTER, GABRIEL (Reed College, Portland, Ore.). Inhibition of growth, synthesis, and permeability in Neurospora crassa by phenethyl alcohol. J. Bacteriol. 90: 29-37. 1965.- Inhibition of the growth of Neurospora crassa in still culture was detected at 0.05% and was complete at a level of 0.2% phenethyl alcohol (PEA). Benzyl alcohol was less in- hibitory, and 3-phenyl-1-propanol and phenol were more inhibitory, than PEA; benzyl- amine and phenethylamine were less inhibitory than the analogous hydroxylated com- pounds. Inhibition by PEA was not reversed by synthetic mixtures of purines and pyrimidines or vitamins, or by casein digests, yeast extract, or nutrient broth. The germination of conidia was inhibited by PEA, but after an exposure of 8.5 hr no loss of viability was observed. The addition of PEA to growing shake cultures caused a simul- taneous inhibition of growth and of the syntheses of ribonucleic and deoxyribonucleic acids and protein; the relationships of these compounds to mycelial dry weight and to one another were constant in growing mycelia, and PEA did not significantly affect these relationships. PEA partially inhibited the uptake of glucose, but severely re- stricted the accumulation of L-leucine, L-tryptophan, or a-aminoisobutyric acid in germinated conidia. The efflux of a-aminoisobutyric acid from germinated conidia was somewhat enhanced by PEA, but this effect was not so pronounced as the (complete) inhibition of a-aminoisobutyric acid accumulation by PEA. -

United States Patent (19) 11 4,267,375 Maasbol Et Al
United States Patent (19) 11 4,267,375 Maasbol et al. 45 May 12, 1981 54 PREPARATION OF THIOETHERS OTHER PUBLICATIONS 75) Inventors: Alfred G. Maasbol, Hamburg, Fed. I. Ruderman et al., J. Amer. Chem. Soc., 71, pp. Rep. of Germany; Lothar G. Dulog, 2264-2265, (1949). St. Martens Latem, Belgium Morrisson and Boyd, Organic Chemistry, 2nd edition, (1967), pp. 29–30. 73) Assignee: s.a. Texaco Belgium in.v., Brussels, T. Todsen et al., J. Amer. Chem. Soc., 72, pp. Belgium 4000-4002, (1950). Berichte Deutsch. Chemie, vol. 1, pp. 587-591, (1935), (21) Appl. No.: 945,273 Berlin. 22 Filed: Sep. 25, 1978 D. Gregg et al., J. Org. Chem., pp. 246-252, (1950). M. Malinovskii, Epoxides and Their Derivatives, pp. Related U.S. Application Data 131-136, (1965), Jerusalem, Daniel Davey & Co. Primary Examiner-Glennon H. Hollrah 63 Continuation of Ser. No. 703,045, Jul. 6, 1976, aban Assistant Examiner-M. C. Eakin doned. Attorney, Agent, or Firm-Carl G. Ries; Robert A. 30 Foreign Application Priority Data Kulason; Carl G. Seutter Nov. 19, 1975 GB United Kingdom ............... 47582/75 57 ABSTRACT .. 51 Int. Cl. ............................................ CO7C 149/30 Thioethers may be prepared by reacting a thiol, such as thiophenol, with an alcohol (having electron donor 52 U.S. C. ......................................... 568/57; 568/58 groups in the alpha or beta position to its hydroxyl 58 Field of Search ........................ 260/609 E, 609 R group) such as phenyl-1-hydroxy-phenethylsulfide. Re 56) References Cited action is carried out in the presence of a Lewis Acid U.S. PATENT DOCUMENTS metal halide, typically zinc chloride. -
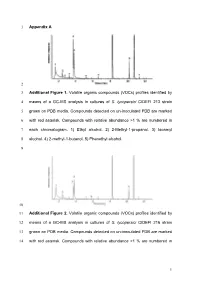
Appendix a 1 2 Additional Figure 1. Volatile Organic Compounds (Vocs
1 Appendix A 2 3 Additional Figure 1. Volatile organic compounds (VOCs) profiles identified by 4 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 213 strain 5 grown on PDB media. Compounds detected on un-inoculated PDB are marked 6 with red asterisk. Compounds with relative abundance >1 % are numbered in 7 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 8 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 9 10 11 Additional Figure 2. Volatile organic compounds (VOCs) profiles identified by 12 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 216 strain 13 grown on PDB media. Compounds detected on un-inoculated PDB are marked 14 with red asterisk. Compounds with relative abundance >1 % are numbered in 1 15 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 16 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 6) Furfuryl alcohol. 17 18 19 Additional Figure 3. Volatile organic compounds (VOCs) profiles identified by 20 means of a GC-MS analysis in cultures of F. fulva CIDEFI 300 strain grown on 21 PDB media. Compounds detected on un-inoculated PDB are marked with red 22 asterisk. Compounds with relative abundance >1 % are numbered in each 23 chromatogram. 6) Furfuryl alcohol. 7) Acetone. 8) Methyl trimethylacetate. 9) 24 Isoamyl alcohol. 10) 1-Octene. 11) 3-Hexanone, 4-methyl-. 12) Styrene. 13) 3- 25 Octanone. 14) Hexanoic acid, 2 ethyl-, methyl ester. 15) 2-Nonanone. 16) 26 Phenethyl alcohol. 17) No identified Nist05. 27 2 28 29 Additional Figure 4. -

Chemicals That Form Explosive Levels of Peroxides Without Concentration (Safe Storage Time After Opening - 3 Months) Chemical CAS # Synonym State Ref
Group A- Chemicals that form explosive levels of peroxides without concentration (Safe storage time after opening - 3 months) Chemical CAS # Synonym State Ref. 000106- Butadiene(1,3) 1,3-Butadiene gas 4 99-0 000126- 2-Chloro-1,3- Chloroprene (1,3) liquid 4 99-8 butadiene 000821- Divinyl acetylene 1,5-Hexadien- 3-yne liquid 5 08-9 000108- Isopropyl ether liquid 5 20-3 000116- Tetrafluoroethylene gas 4 14-3 000109- Vinyl ether Divinyl ether liquid 5 93-3 000075- 1,1- Vinylidene chloride liquid 5 35-4 Dichloroethylene Group B-Chemicals that form explosive levels of peroxides on concentration (Safe storage time after opening - 12 months) Chemical CAS # Synonym State Ref. 000105- Acetal liquid 5 57-7 000075- Acetaldehyde liquid 4 07-0 000100- Benzyl alcohol liquid 4 51-6 000078- 2-Butanol liquid 4 92-2 000108- Cyclohexanol liquid 4 93-0 000110- Cyclohexene liquid 5 83-8 000822- 2-Cyclohexen-1-ol liquid 4 67-3 000142- Cyclopentene liquid 5 29-0 000091- Decahydronaphthalene liquid 4 17-8 000460- Diacetylene gas 5 12-8 000077- Dicyclopentadiene liquid 5 73-6 Diethylene glycol 000111- Diglyme liquid 5 dimethyl ether 96-6 000123- Dioxane 1,4-Dioxane liquid 5 91-1 Ethylene glycol 000110- Glyme liquid 5 dimethyl ether 71-4 000060- Ethyl ether Diethyl ether liquid 5 29-7 000110- Furan liquid 5 00-9 000589- 4-Heptanol liquid 4 55-9 000626- 2-Hexanol liquid 4 93-7 000098- Isopropyl benzene Cumene liquid 5 82-8 000074- Methyl acetylene Propyne gas 5 99-7 000123- 3-Methyl-1-butanol Isoamyl alcohol liquid 4 51-3 000096- Methyl cyclopentane liquid 5 37-7 -

CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic Anhydride Liquid
CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic anhydride liquid Acetaminophen Synthesis p-aminophenol solid Alcohols to Alkyl chlorides 2-pentanol liquid Alcohols to Alkyl chlorides Hydrochloric acid 12 M Alcohols to Alkyl chlorides Sodium carbonate solid Alcohols to Alkyl chlorides Hydrobromic acid 48% w/v Alcohols to Alkyl chlorides Sodium sulfate anhydrous solid Alcohols to Alkyl chlorides sec-phenethyl alcohol liquid Alcohols to Alkyl chlorides Benzyl alcohol liquid Alcohols to Alkyl chlorides t-butanol liquid Alcohols to Alkyl chlorides 1-pentanol liquid Alcohols to Alkyl chlorides Sodium carbonate 10% w/v Diels Alder Reaction 2,3-dimethyl-1,3-butadiene liquid Diels Alder Reaction Maleic anhydride solid Diels Alder Reaction Ethanol 95% Liquid Diels Alder Reaction Hexane liquid Diels Alder Reaction Cyclohexane liquid Diels Alder Reaction Calcium chloride solid Esterification methanol liquid Esterification Sodium carbonate 10% w/v Esterification 1-propanol liquid Esterification 1-butanol liquid Esterification trans-cinnamic acid solid Esterification Isoamyl alcohol liquid Esterification Isopropyl alcohol liquid Esterification Benzyl alcohol liquid Esterification Sulfuric acid conc. 18 M Esterification 1-pentanol liquid Esterification Isobutyl alcohol liquid Esterification Ethanol 95% liquid Page 1 of 3 Experiment Title Chemical Name Concentration Extraction of Beta Carotene Cyclohexane liquid Extraction of Beta Carotene Beta carotene UV -

Phenethyl Alcohol Is an Effective Non-Traditional Preservative Agent for Cosmetic Preparations
Online - 2455-3891 Vol 10, Issue 8, 2017 Print - 0974-2441 Research Article PHENETHYL ALCOHOL IS AN EFFECTIVE NON-TRADITIONAL PRESERVATIVE AGENT FOR COSMETIC PREPARATIONS SASITHORN SIRILUN1, CHAIYAVAT CHAIYASUT1, BHAGAVATHI SUNDARAM SIVAMARUTHI1, SARTJIN PEERAJAN2, NAPHATSORN KUMAR3, PERIYANAINA KESIKA1* 1Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand. 2Health Innovation Institute, Chiang Mai 50200, Thailand. 3School of Cosmetic Science, Mae Fah Luang University, Chiang Rai 57100, Thailand. Email: [email protected]; [email protected] Received: 19 March 2017, Revised and Accepted: 26 April 2017 ABSTRACT Objective: Preservatives are used in the cosmetic products to protect the potential growth of microbes, therefore, to prolong the shelf life of products, and to protect the consumer from infections. However, several preservatives can cause various health problems, and the safety profiles of those preservatives are still unclear. Many natural substances are used in the cosmetic products to substitute the traditional preservatives. The present study deals with the evaluation of conservative nature of phenethyl alcohol (PEA) in three cosmetic formulations (emulsion, cleansing, and conditioner). Methods: Three different concentrations of PEA (0.3%, 1%, and 2.5%) were used in cosmetic formulations. The physical appearance of the formulas was assessed manually, and the antimicrobial nature of PEA and PEA-containing cosmetic formulations was evaluated by agar well plate assay. Results: The use of PEA has not affected the physical appearance and quality of the formulations, except the high concentration of PEA in the cleansing solution, which reduced the foam formation. The minimal required concentration of PEA in emulsions and cleansings was 1.0% and 2.5% in the conditioners. -

Catalysed Nucleophilic Addition of Alcohols to Cyclopropenes
Chapter 3 – Gold(I)-Catalysed Nucleophilic Addition of Alcohols to Cyclopropenes Acknowledgment: Results presented in this chapter was carried out together with J.T. Bauer, who performed the reactions in Table 1 (Entries 1-11) and Table 4. All other reactions presented in this chapter were the work of the author. Development of Gold-Catalysed Reactions – Gold(I)-Catalysed Nucleophilic Addition of Alcohols to Cyclopropenes 3.1 Introduction Since the 1850’s when Alexander Williamson first developed the synthesis of ethers1 the process has become vital in both research and industry alike. Although the Williamson ether synthesis reaction is the most widely used in the formation of ethers, the harsh basic conditions and often high temperatures can lead to undesirable side reactions. Further complications can arise upon the use of more sterically encumbered reagents which will either prove to be unreactive, or when attempting to form tertiary ethers, the elimination pathway is more highly favoured.2, 3 Figure 1 Tertiary Alkyl Allylic Ether Thus, alkyl-tertiary allylic ethers (3.1, Figure 1) are difficult to make via conventional methods. Although tertiary allylic alcohols are easy to access (via vinyl Grignard addition to ketones, for example), attempted etherification of the corresponding sterically hindered alkoxides (with an alkyl halide) tends to lead to the elimination rather than the substitution pathway. Figure 2 Vinylglycidol A possible route to access tert-allylic ethers was devised by Trost et. al. as they were attempting to enantioselectively -

Powerful Promoters for Ring-Opening Reactions of Epoxides with Carbon Nucleophiles
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2021, part iii, 0-0 Organic Chemistry to be inserted by editorial office Fluorinated alcohols: powerful promoters for ring-opening reactions of epoxides with carbon nucleophiles Taylor L. Dover and Frank E. McDonald* Department of Chemistry, Emory University, 1515 Dickey Drive NE, Atlanta, GA 30322, USA Email: [email protected] In honor of the 70th birthday of our colleague and mentor, Professor Lanny S. Liebeskind Received mm-dd-yyyy Accepted mm-dd-yyyy Published on line mm-dd-yyyy Dates to be inserted by editorial office Abstract Ring-opening reactions of epoxides with carbon nucleophiles are valuable transformations for constructing functionalized carbon-carbon bonds. Epoxide ring-opening methods typically require Lewis acidic additives and/or strong nucleophiles to overcome the activation barrier for these reactions. Fluorinated alcohol solvents present a desirable alternative, enhancing the efficacy of these reactions with weak and neutral carbon nucleophiles by promoting electrophilic activation of the epoxide. We present here a thorough review of the literature regarding epoxide ring-opening reactions with carbon nucleophiles in fluorinated alcohol solvents, concluding with a few recent examples with aziridines. Keywords: Carbon nucleophiles, epoxides, fluorinated alcohols, ring-opening DOI: https://doi.org/10.24820/ark.5550190.p011.449 Page 1 ©AUTHOR(S) Arkivoc 2021, iii, 0-0 Dover, T. L. et al. Table of Contents 1. Introduction 1.1 General 1.2 Properties of fluorinated alcohol solvents 2. Ring-opening Reactions with Neutral Carbon Nucleophiles, with Fluorinated Alcohol Solvents as Substitutes for Lewis acid Promoters or Catalysts 2.1 Intermolecular carbon-carbon bond-forming reactions 2.2 Intramolecular carbon-carbon bond-forming reactions 3. -

United States Patent (19) 4,731,488
United States Patent (19) 11 Patent Number: 4,731,488 Shimasaki et al. 45) Date of Patent: Mar. 15, 1988 54, WAPOR-PHASE HYDROGEN TRANSFER 4,255,283 3/1981 Bartek et al. ........................ 502/202 REACTION OF CARBONYL COMPOUND 4,407,732 10/1983 Kehl .................................... 502/208 4,418,005 11/1983 Dodd et al. ..................... 502/340 X WTH ALCOHOL 4,471,070 9/1984 Siefert et al. ................... 502/341X (75) Inventors: Yuuji Shimasaki, Suita; Youichi Hino, Sakai; Michio Ueshima, FOREIGN PATENT DOCUMENTS Takarazuka, all of Japan 5549322 10/1978 Japan ................................... 502/.340 73 Assignee: Nippon Shokubai Kagaku Kogyo Co., 727209 8/1977 U.S.S.R. .............................. 502/.340 Ltd., Osaka, Japan OTHER PUBLICATIONS (21) Appl. No.: 873,447 . Selective Reduction of Unsaturated Aldehydes and Ketones by Vapor-Phase Hydrogen Transfer Reaction, (22 Filed: Jun. 6, 1986 Ballard et al., Shell Development Co., Emeryville, Related U.S. Application Data Calif., pp. 754-763, Mar. 16, 1956. 62 Division of Ser. No. 802,183, Nov. 25, 1985, aban Primary Examiner-J. E. Evans doned. Attorney, Agent, or Firm-Sherman and Shalloway 30 Foreign Application Priority Data (57) ABSTRACT Nov. 27, 1984 JP Japan ................................ 59-2486.50 A catalyst for vapor-phase hydrogen transfer reaction Apr. 8, 1985 JP Japan .................................. 60-72698 between a carbonyl compound having an alkenyl or aryl group and a primary or secondary alcohol, said 51 Int. Cl." .................... C07C29/136; CO7C29/14; catalyst having a composition represented by the fol C07C 33/03; CO7C 33/22 52 U.S. C. .................................... 568/648; 502/202; lowing general formula 502/242; 502/303; 502/341; 568/813; 568/814; 568/825; 568/881 MgaxiY-Od 58) Field of Search .............. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Sandalwood, white (yellow, or East Indian) ... Santalum album L. Sandarac ........................................................ Tetraclinis articulata (Vahl.), Mast .............................. In alcoholic beverages only Sarsaparilla ..................................................... Smilax aristolochiaefolia Mill., (Mexican sarsaparilla), S. regelii Killip et Morton (Honduras sarsaparilla), S. febrifuga Kunth (Ecuadorean sarsaparilla), or undetermined Smilax spp. (Ecuadorean or Central American sarsaparilla). Sassafras leaves ............................................ Sassafras albidum (Nutt.) Nees ................................. Safrole free Senna, Alexandria .......................................... Cassia acutifolia Delile. Serpentaria (Virginia snakeroot) .................... Aristolochia serpentaria L ........................................... In alcoholic beverages only Simaruba bark ................................................ Simaruba amara Aubl ................................................. Do. Snakeroot, Canadian (wild ginger) ................ Asarum canadense L. Spruce needles and twigs .............................. Picea glauca (Moench) Voss or P. mariana (Mill.) BSP. Storax (styrax) ................................................ Liquidambar orientalis Mill. or L. styraciflua L. Tagetes (marigold) ......................................... Tagetes patula L., T. erecta L., or T. minuta L. (T. As oil only glandulifera