VPINP723 Ingredient Guide Update for Approval
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,168,397 B2 Amalric Et Al
US009 168397B2 (12) United States Patent (10) Patent No.: US 9,168,397 B2 Amalric et al. (45) Date of Patent: Oct. 27, 2015 (54) POWDERY EMULSIFYING COMPOSITION A61Q 19/005; A61Q 1/02: A61K 8/92: OF ALKYL POLYGLYCOSIDES, USE A61K 8/34: A61K 8/602: A61K 8/022: THEREOF FOR PREPARING COSMETC A61K 8/60; A61K 8/062: A61K 8/604; EMULSIONS, AND METHOD FOR A61K 8/06; A61K 8/342: A61K 2800/412: PREPARING SAME BO1F17/0092 See application file for complete search history. (71) Applicant: SOCIETE DEXPLOITATION DE PRODUITS POUR LES INDUSTRIES CHIMIQUES SEPPIC, (56) References Cited Paris (FR) U.S. PATENT DOCUMENTS (72) Inventors: Chantal Amalric, Blan (FR); Alicia Roso, Saix (FR); Agnes Gorce, 5,670471 A 9, 1997 Amalric et al. 5,888,482 A 3, 1999 Amalric et al. Marseille (FR) 5,958,431 A 9/1999 Brancq et al. 6,245,821 B1 6/2001 Bulcourt et al. (73) Assignee: SOCIETE DEXPLOITATION DE 6,268.400 B1 7/2001 Amalric et al. PRODUITS POUR LES 6,353,034 B1 3/2002 Amalric et al. INDUSTRIES CHIMIQUES SEPPIC, 6,488,946 B1 12/2002 Milius et al. Paris (FR) 6,723,774 B2 4/2004 Guntherberg et al. 2003/0105169 A1 6/2003 Lennon (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. EP O992508 4/2000 FR 2756195 5, 1998 (21) Appl. No.: 14/278,908 FR 2784904 4/2000 FR 283O464 4/2003 (22) Filed: May 15, 2014 WO 92O6778 4f1992 WO 9513863 5, 1995 (65) Prior Publication Data WO 9637285 11 1996 WO 9637286 11, 1996 US 2014/O2554.57 A1 Sep. -

Hydrogenation of Styrene Oxide to 2-Phenylethanol Over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method
Hindawi Publishing Corporation International Journal of Chemical Engineering Volume 2014, Article ID 406939, 6 pages http://dx.doi.org/10.1155/2014/406939 Research Article Hydrogenation of Styrene Oxide to 2-Phenylethanol over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method Sunil K. Kanojiya,1 G. Shukla,1 S. Sharma,1 R. Dwivedi,2 P. Sharma,2 R. Prasad,2 M. Satalkar,3 and S. N. Kane3 1 IPCA Laboratories Ltd., Indore 452003, India 2 School of Chemical Sciences, DA University, Indore 452001, India 3 Magnetic Materials Laboratory, School of Physics, DA University, Indore 452001, India Correspondence should be addressed to R. Prasad; [email protected] Received 14 February 2014; Accepted 20 May 2014; Published 17 July 2014 Academic Editor: Moshe Sheintuch Copyright © 2014 Sunil K. Kanojiya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Nanocrystalline nickel prepared by glycol reduction method and characterized by XRD and magnetic measurements has been used as a catalyst for hydrogenation of styrene oxide to 2-phenylethanol. Effect of process variables such as particle size of the catalyst, temperature, and pressure have been optimized to achieve a maximum conversion of 98% of styrene oxide with 99% selectivity towards 2-phenylethanol. The structure of the transition state has been computed employing density functional theory −1 and using Gaussian 09 suite. The enthalpy of reaction (Δ) and activation energy ()arecalculatedtobe85.3kcal⋅mol and −1 123.03 kcal⋅mol , respectively. A tentative mechanism for the reaction is proposed according to which atomized hydrogen and styrene oxide react together over the catalyst surface to produce 2-phenylethanol. -

USP Excipient Reference Standards Catalog
Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) 1002505 Acesulfame G0H082 F0C136 (30-JUN- 55589-62-3 N/A $410.00 Potassium (200 mg) 2009) 1005706 Glacial Acetic Acid R038A0 I0M342 (31-JUL- 64-19-7 N/A $280.00 (1.5 mL/ampule; 3 2017) ampules) 1006801 Acetone (1.5 R095B0 I0M548 (28-FEB- 67-64-1 N/A $265.00 mL/ampule; 3 2022) ampules) 1009005 Acetylcysteine (200 R10200 K0K294 (30- 616-91-1 N/A $245.00 mg) NOV-2019) 1009901 Acetyltributyl Citrate R053A0 H0I337 (31-AUG- 77-90-7 N/A $275.00 (3 x 200 mg) 2017) 1009923 Acetyltriethyl Citrate R041M0 H0I339 (31-JAN- 77-89-4 N/A $275.00 (500 mg) 2017) 1012190 Adipic Acid (100 mg) F1D318 F0D318 (31- 124-04-9 N/A $265.00 MAR-2018) 1012214 Agar (500 mg) F0K137 N/A N/A $253.00 1012595 rAlbumin Human (6 R04600 G0M268 (31- N/A N/A $314.00 Cold Shipment mg) (Recombinant DEC-2016) Required Human Albumin) (COLD SHIPMENT REQUIRED) 1012688 Alcohol R05900 G0M024 (31- 64-17-5 N/A $265.00 Determination-- OCT-2018) Alcohol (5 Page 1 Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) mL/ampule; 5 ampules) 1012768 Alcohol (1.2 R127S0 R087W0 (31- 64-17-5 N/A $245.00 mL/ampule; 5 MAY-2020) ampules) 1012772 Dehydrated Alcohol R119J0 G0M292 (31- 64-17-5 N/A $245.00 (1.2 mL/ampule; 5 MAY-2020) ampules) 1012799 Aleuritic Acid (50 F02300 533-87-9 N/A $278.00 mg) -

Green Chemistry : Greener Alternatives to Synthetic Organic Transformations
Green Chemistry Greener Alternatives to Synthetic Organic Transformations V.K. Ahluwalia Alpha Science International Ltd. Oxford, U.K. Contents Preface v Parti 1. Introduction 1.1-1.10 1.1 Principles of Green Chemistry 1.1 1.2 How to Plan a Green Synthesis 1.2 Part II Green Alternatives to Synthesis Organic Transformations 2. Aqueous Phase Transformations 2.1-2.48 2.1 p-Acetylaminophenol (Tylenol) 2.1 2.2 3-Aminopyridine 2.2 2.2a Anthranilic Acid 2.3 2.3 Benzilic Acid 2.3 2.4 Benzoin 2.5 2.5 Benzotriazole 2.6 2.6 2-Benzoyl-3,5-dimethylbenzofuran 2.7 2.7 n-Butyl Bromide 2.8 2.8 Tert.Butylchloride 2.8 2.9 Chalcone (Benzalacetophenone) 2.9 2.10 Cycloheptanone 2.10 2.11 2,3-Dihydroxy Anisole (Pyrigallol Monomethyl-ether) 2.12 2.12 2,4-Dihydroxybenzoic acid (P-resorcylic Acid) 2.13 2.13 3,4-Dimethoxyphenol 2.14 2.14 2,3-Dimethyl-l-phenylpyrazol-5-one 2.15 2.15 3, 5-Dimethylpyrazole 2.16 2.16 5,5-Diphenylhydantoin 2.17 2.17 Endo-cis-1,4-endoxo-A5-cyclohexene-2,3-dicarboxylic Acid 2.18 2.18 p-Ethoxyacetanilide (Phenacetin) 2.19 2.19 6-Ethoxycarbonyl-3,5-diphenyI-2-cyclohexenone 2.20 2.20 HeteroDiels-AlderAdduct 2.22 2.21 Hippuric Acid (Benzoyl Glycine) 2.22 Viii Contents 2.22 Hydantion 2.23 2.25 3-Hydroxy-3-phenyl-2-methylene Proponamide 2.24 2.24 Iodoform 2.25 2.25 Inodole 2.26 2.26 3-(p-Methoxyphenyl)-2H-1,4-Benzoxazine 2.27 2.27 3-Methylcyclopent-2-enone 2.27 2.28 2-(2'-Methlindol-3yl)-1,4-benzoquinone 2.28 2.29 2-methyl-2-(3-oxobutyl)-1,3-cyclopentanedione 2.29 2.30 P-Naphthyl Acetate 2.30 2.31 (5-Naphthyl Methyl Ether (Nerolin) 2.31 2.32 -

Inhibition of Growth, Synthesis, and Permeability in Neurospora Crassa by Phenethyl Alcohol
JOURNAL OF BACTERIOLOGY, JUly, 1965 Vol. 90, No. I Copyright © 1965 American Society for Microbiology Printed in U.S.A. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol GABRIEL LESTER Department of Biology, Reed College, Portland, Oregon Received for publication 22 January 1965 ABSTRACT LESTER, GABRIEL (Reed College, Portland, Ore.). Inhibition of growth, synthesis, and permeability in Neurospora crassa by phenethyl alcohol. J. Bacteriol. 90: 29-37. 1965.- Inhibition of the growth of Neurospora crassa in still culture was detected at 0.05% and was complete at a level of 0.2% phenethyl alcohol (PEA). Benzyl alcohol was less in- hibitory, and 3-phenyl-1-propanol and phenol were more inhibitory, than PEA; benzyl- amine and phenethylamine were less inhibitory than the analogous hydroxylated com- pounds. Inhibition by PEA was not reversed by synthetic mixtures of purines and pyrimidines or vitamins, or by casein digests, yeast extract, or nutrient broth. The germination of conidia was inhibited by PEA, but after an exposure of 8.5 hr no loss of viability was observed. The addition of PEA to growing shake cultures caused a simul- taneous inhibition of growth and of the syntheses of ribonucleic and deoxyribonucleic acids and protein; the relationships of these compounds to mycelial dry weight and to one another were constant in growing mycelia, and PEA did not significantly affect these relationships. PEA partially inhibited the uptake of glucose, but severely re- stricted the accumulation of L-leucine, L-tryptophan, or a-aminoisobutyric acid in germinated conidia. The efflux of a-aminoisobutyric acid from germinated conidia was somewhat enhanced by PEA, but this effect was not so pronounced as the (complete) inhibition of a-aminoisobutyric acid accumulation by PEA. -

United States Patent (19) 11 4,267,375 Maasbol Et Al
United States Patent (19) 11 4,267,375 Maasbol et al. 45 May 12, 1981 54 PREPARATION OF THIOETHERS OTHER PUBLICATIONS 75) Inventors: Alfred G. Maasbol, Hamburg, Fed. I. Ruderman et al., J. Amer. Chem. Soc., 71, pp. Rep. of Germany; Lothar G. Dulog, 2264-2265, (1949). St. Martens Latem, Belgium Morrisson and Boyd, Organic Chemistry, 2nd edition, (1967), pp. 29–30. 73) Assignee: s.a. Texaco Belgium in.v., Brussels, T. Todsen et al., J. Amer. Chem. Soc., 72, pp. Belgium 4000-4002, (1950). Berichte Deutsch. Chemie, vol. 1, pp. 587-591, (1935), (21) Appl. No.: 945,273 Berlin. 22 Filed: Sep. 25, 1978 D. Gregg et al., J. Org. Chem., pp. 246-252, (1950). M. Malinovskii, Epoxides and Their Derivatives, pp. Related U.S. Application Data 131-136, (1965), Jerusalem, Daniel Davey & Co. Primary Examiner-Glennon H. Hollrah 63 Continuation of Ser. No. 703,045, Jul. 6, 1976, aban Assistant Examiner-M. C. Eakin doned. Attorney, Agent, or Firm-Carl G. Ries; Robert A. 30 Foreign Application Priority Data Kulason; Carl G. Seutter Nov. 19, 1975 GB United Kingdom ............... 47582/75 57 ABSTRACT .. 51 Int. Cl. ............................................ CO7C 149/30 Thioethers may be prepared by reacting a thiol, such as thiophenol, with an alcohol (having electron donor 52 U.S. C. ......................................... 568/57; 568/58 groups in the alpha or beta position to its hydroxyl 58 Field of Search ........................ 260/609 E, 609 R group) such as phenyl-1-hydroxy-phenethylsulfide. Re 56) References Cited action is carried out in the presence of a Lewis Acid U.S. PATENT DOCUMENTS metal halide, typically zinc chloride. -
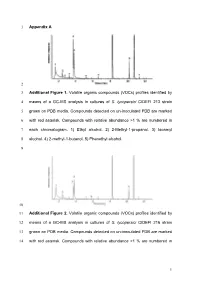
Appendix a 1 2 Additional Figure 1. Volatile Organic Compounds (Vocs
1 Appendix A 2 3 Additional Figure 1. Volatile organic compounds (VOCs) profiles identified by 4 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 213 strain 5 grown on PDB media. Compounds detected on un-inoculated PDB are marked 6 with red asterisk. Compounds with relative abundance >1 % are numbered in 7 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 8 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 9 10 11 Additional Figure 2. Volatile organic compounds (VOCs) profiles identified by 12 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 216 strain 13 grown on PDB media. Compounds detected on un-inoculated PDB are marked 14 with red asterisk. Compounds with relative abundance >1 % are numbered in 1 15 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 16 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 6) Furfuryl alcohol. 17 18 19 Additional Figure 3. Volatile organic compounds (VOCs) profiles identified by 20 means of a GC-MS analysis in cultures of F. fulva CIDEFI 300 strain grown on 21 PDB media. Compounds detected on un-inoculated PDB are marked with red 22 asterisk. Compounds with relative abundance >1 % are numbered in each 23 chromatogram. 6) Furfuryl alcohol. 7) Acetone. 8) Methyl trimethylacetate. 9) 24 Isoamyl alcohol. 10) 1-Octene. 11) 3-Hexanone, 4-methyl-. 12) Styrene. 13) 3- 25 Octanone. 14) Hexanoic acid, 2 ethyl-, methyl ester. 15) 2-Nonanone. 16) 26 Phenethyl alcohol. 17) No identified Nist05. 27 2 28 29 Additional Figure 4. -

Chemicals That Form Explosive Levels of Peroxides Without Concentration (Safe Storage Time After Opening - 3 Months) Chemical CAS # Synonym State Ref
Group A- Chemicals that form explosive levels of peroxides without concentration (Safe storage time after opening - 3 months) Chemical CAS # Synonym State Ref. 000106- Butadiene(1,3) 1,3-Butadiene gas 4 99-0 000126- 2-Chloro-1,3- Chloroprene (1,3) liquid 4 99-8 butadiene 000821- Divinyl acetylene 1,5-Hexadien- 3-yne liquid 5 08-9 000108- Isopropyl ether liquid 5 20-3 000116- Tetrafluoroethylene gas 4 14-3 000109- Vinyl ether Divinyl ether liquid 5 93-3 000075- 1,1- Vinylidene chloride liquid 5 35-4 Dichloroethylene Group B-Chemicals that form explosive levels of peroxides on concentration (Safe storage time after opening - 12 months) Chemical CAS # Synonym State Ref. 000105- Acetal liquid 5 57-7 000075- Acetaldehyde liquid 4 07-0 000100- Benzyl alcohol liquid 4 51-6 000078- 2-Butanol liquid 4 92-2 000108- Cyclohexanol liquid 4 93-0 000110- Cyclohexene liquid 5 83-8 000822- 2-Cyclohexen-1-ol liquid 4 67-3 000142- Cyclopentene liquid 5 29-0 000091- Decahydronaphthalene liquid 4 17-8 000460- Diacetylene gas 5 12-8 000077- Dicyclopentadiene liquid 5 73-6 Diethylene glycol 000111- Diglyme liquid 5 dimethyl ether 96-6 000123- Dioxane 1,4-Dioxane liquid 5 91-1 Ethylene glycol 000110- Glyme liquid 5 dimethyl ether 71-4 000060- Ethyl ether Diethyl ether liquid 5 29-7 000110- Furan liquid 5 00-9 000589- 4-Heptanol liquid 4 55-9 000626- 2-Hexanol liquid 4 93-7 000098- Isopropyl benzene Cumene liquid 5 82-8 000074- Methyl acetylene Propyne gas 5 99-7 000123- 3-Methyl-1-butanol Isoamyl alcohol liquid 4 51-3 000096- Methyl cyclopentane liquid 5 37-7 -

CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic Anhydride Liquid
CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic anhydride liquid Acetaminophen Synthesis p-aminophenol solid Alcohols to Alkyl chlorides 2-pentanol liquid Alcohols to Alkyl chlorides Hydrochloric acid 12 M Alcohols to Alkyl chlorides Sodium carbonate solid Alcohols to Alkyl chlorides Hydrobromic acid 48% w/v Alcohols to Alkyl chlorides Sodium sulfate anhydrous solid Alcohols to Alkyl chlorides sec-phenethyl alcohol liquid Alcohols to Alkyl chlorides Benzyl alcohol liquid Alcohols to Alkyl chlorides t-butanol liquid Alcohols to Alkyl chlorides 1-pentanol liquid Alcohols to Alkyl chlorides Sodium carbonate 10% w/v Diels Alder Reaction 2,3-dimethyl-1,3-butadiene liquid Diels Alder Reaction Maleic anhydride solid Diels Alder Reaction Ethanol 95% Liquid Diels Alder Reaction Hexane liquid Diels Alder Reaction Cyclohexane liquid Diels Alder Reaction Calcium chloride solid Esterification methanol liquid Esterification Sodium carbonate 10% w/v Esterification 1-propanol liquid Esterification 1-butanol liquid Esterification trans-cinnamic acid solid Esterification Isoamyl alcohol liquid Esterification Isopropyl alcohol liquid Esterification Benzyl alcohol liquid Esterification Sulfuric acid conc. 18 M Esterification 1-pentanol liquid Esterification Isobutyl alcohol liquid Esterification Ethanol 95% liquid Page 1 of 3 Experiment Title Chemical Name Concentration Extraction of Beta Carotene Cyclohexane liquid Extraction of Beta Carotene Beta carotene UV -

OLEYL ALCOHOL Alcohol Oleicus OLIVE LEAF Oleae Folium
Oleyl alcohol EUROPEAN PHARMACOPOEIA 5.0 — arachidic acid: not more than 2.0 per cent, Composition of fatty alcohols. Gas chromatography — eicosenoic acid:notmorethan2.0percent. (2.2.28): use the normalisation procedure. Ethylene oxide and dioxan (2.4.25). Not more than 1 ppm Test solution. Mix 25 mg of the substance to be examined of ethylene oxide and 10 ppm of dioxan. with 1.0 ml of methylene chloride R. Heavy metals (2.4.8). 2.0 g complies with limit test C for Reference solution (a). Dissolve 25 mg of each of arachidyl heavy metals (10 ppm). Prepare the standard using 2 ml of alcohol R, linolenyl alcohol R, linoleyl alcohol R, oleyl lead standard solution (10 ppm Pb) R. alcohol R, palmityl alcohol R and stearyl alcohol R in methylene chloride R and dilute to 5 ml with the same Water (2.5.12). Not more than 1.0 per cent, determined solvent. Dilute 1 ml of this solution to 5 ml with methylene on 1.0 g by the semi-micro determination of water. Use chloride R. amixtureof30volumesofanhydrous methanol R and Reference solution (b). Dissolve 10 mg of linoleyl alcohol R 70 volumes of methylene chloride R as solvent. and 1 g of oleyl alcohol R in methylenechlorideRand Total ash (2.4.16). Not more than 0.1 per cent, determined dilute to 40 ml with the same solvent. on 1.0 g. Column: STORAGE — size: l =30m,Ø=0.32mm, Store protected from light and at room temperature. — stationary phase: poly(dimethyl)siloxane R (film thickness 1 µm). -

Phenethyl Alcohol Is an Effective Non-Traditional Preservative Agent for Cosmetic Preparations
Online - 2455-3891 Vol 10, Issue 8, 2017 Print - 0974-2441 Research Article PHENETHYL ALCOHOL IS AN EFFECTIVE NON-TRADITIONAL PRESERVATIVE AGENT FOR COSMETIC PREPARATIONS SASITHORN SIRILUN1, CHAIYAVAT CHAIYASUT1, BHAGAVATHI SUNDARAM SIVAMARUTHI1, SARTJIN PEERAJAN2, NAPHATSORN KUMAR3, PERIYANAINA KESIKA1* 1Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand. 2Health Innovation Institute, Chiang Mai 50200, Thailand. 3School of Cosmetic Science, Mae Fah Luang University, Chiang Rai 57100, Thailand. Email: [email protected]; [email protected] Received: 19 March 2017, Revised and Accepted: 26 April 2017 ABSTRACT Objective: Preservatives are used in the cosmetic products to protect the potential growth of microbes, therefore, to prolong the shelf life of products, and to protect the consumer from infections. However, several preservatives can cause various health problems, and the safety profiles of those preservatives are still unclear. Many natural substances are used in the cosmetic products to substitute the traditional preservatives. The present study deals with the evaluation of conservative nature of phenethyl alcohol (PEA) in three cosmetic formulations (emulsion, cleansing, and conditioner). Methods: Three different concentrations of PEA (0.3%, 1%, and 2.5%) were used in cosmetic formulations. The physical appearance of the formulas was assessed manually, and the antimicrobial nature of PEA and PEA-containing cosmetic formulations was evaluated by agar well plate assay. Results: The use of PEA has not affected the physical appearance and quality of the formulations, except the high concentration of PEA in the cleansing solution, which reduced the foam formation. The minimal required concentration of PEA in emulsions and cleansings was 1.0% and 2.5% in the conditioners. -

Ingredient Book Content
Beauty Care Ingredient Book Content Sustainability 2 Our Technologies 4 Active Beauty Actives 6 Inspiration, Innovation Skin Care solutions at a glance 8 Anti-aging 10 Connection, everything Daily defense 15 Skin complexion 22 Skin balance 24 with Passion Moisturization 27 Skin relief 29 Body shape 32 Hair Care solutions at a glance 34 By your side throughout the world, SEPPIC Beauty Care imagines Hair & Scalp care 36 & develops ingredients for Active Beauty (Proven efficacy & Marine extracts 39 attractive concepts) and for Sensorial Beauty (Unique performances & creative textures), to inspire the cosmetics of tomorrow. Sensorial Beauty Polymeric Thickeners & Stabilizers 42 Emulsifiers 49 Sensory Ingredients 55 Emollients 56 Waxes 58 Powders 58 Functional Additives 60 Foaming & solubilizing agents 62 Scrubs 65 Preservatives & boosters 66 Tables Skin biology 20 Hair biology 35 Beauty concepts 40 Expert solutions 67 A-Z Index 68 Abreviation list 74 Legend of pictograms 75 1 Corporate Social INNOVATION Our 2020 objective: 100% of our New Products bring at least one Environmental or Health benefit Responsibility Design new product with at least one environmental or health benefit for people, regarding one of the following criterias: Raw materials, renewable, bio-sourced or sustainably sourced or derived from green chemistry, sustainability of procurement ENVIRONMENTAL Manufacture product according to green chemistry principles Our 2020 objective: Customer’s process with a lower environmental impact (lower consumption of energy or water, lower