Food and Drug Administration, HHS § 172.515
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrogenation of Styrene Oxide to 2-Phenylethanol Over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method
Hindawi Publishing Corporation International Journal of Chemical Engineering Volume 2014, Article ID 406939, 6 pages http://dx.doi.org/10.1155/2014/406939 Research Article Hydrogenation of Styrene Oxide to 2-Phenylethanol over Nanocrystalline Ni Prepared by Ethylene Glycol Reduction Method Sunil K. Kanojiya,1 G. Shukla,1 S. Sharma,1 R. Dwivedi,2 P. Sharma,2 R. Prasad,2 M. Satalkar,3 and S. N. Kane3 1 IPCA Laboratories Ltd., Indore 452003, India 2 School of Chemical Sciences, DA University, Indore 452001, India 3 Magnetic Materials Laboratory, School of Physics, DA University, Indore 452001, India Correspondence should be addressed to R. Prasad; [email protected] Received 14 February 2014; Accepted 20 May 2014; Published 17 July 2014 Academic Editor: Moshe Sheintuch Copyright © 2014 Sunil K. Kanojiya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Nanocrystalline nickel prepared by glycol reduction method and characterized by XRD and magnetic measurements has been used as a catalyst for hydrogenation of styrene oxide to 2-phenylethanol. Effect of process variables such as particle size of the catalyst, temperature, and pressure have been optimized to achieve a maximum conversion of 98% of styrene oxide with 99% selectivity towards 2-phenylethanol. The structure of the transition state has been computed employing density functional theory −1 and using Gaussian 09 suite. The enthalpy of reaction (Δ) and activation energy ()arecalculatedtobe85.3kcal⋅mol and −1 123.03 kcal⋅mol , respectively. A tentative mechanism for the reaction is proposed according to which atomized hydrogen and styrene oxide react together over the catalyst surface to produce 2-phenylethanol. -

Hazardous Substance Fact Sheet
Right to Know Hazardous Substance Fact Sheet Common Name: DIOXOLANE Synonyms: 1,3-Dioxolan; Formal Glycol; Glycol Methylene Ether CAS Number: 646-06-0 Chemical Name: 1,3-Dioxolane RTK Substance Number: 0791 Date: February 1999 Revision: April 2008 DOT Number: UN 1166 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE Dioxolane is a clear, colorless liquid with an Ether-like odor. It Hazard Summary is used in lithium batteries and as a solvent for oils, fats, waxes Hazard Rating NJDOH NFPA and dyes. HEALTH - 1 FLAMMABILITY - 3 REACTIVITY - 2 FLAMMABLE AND REACTIVE POISONOUS GASES ARE PRODUCED IN FIRE CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f Dioxolane is on the Right to Know Hazardous Substance List because it is cited by ACGIH, DOT, and NFPA. f Dioxolane can affect you by ingestion and may be absorbed f This chemical is on the Special Health Hazard Substance through the skin. List. f Contact can irritate and burn the skin and eyes with possible eye damage. f Inhaling Dioxolane can irritate the nose and throat causing coughing and wheezing. f Exposure to Dioxolane may damage the nervous system. f Dioxolane may damage the kidneys. f Dioxolane is FLAMMABLE and REACTIVE and a SEE GLOSSARY ON PAGE 5. DANGEROUS FIRE and EXPLOSION HAZARD. FIRST AID Eye Contact f Immediately flush with large amounts of cool water for at Workplace Exposure Limits least 15 minutes, lifting upper and lower lids. Remove ACGIH: The threshold limit value (TLV) is 20 ppm averaged contact lenses, if worn, while flushing. -

A Review on Electrochemical Properties of Choline Chloride Based Eutectic Solvent in Mineral Processing
JASEM ISSN 1119 -8362 Full-text Available Online at J. Appl. Sci. Environ. Manage. August 2017 Vol. 21 (5) 991-998 www.ajol.info and All rights reserved www.bioline.org.br/ja A Review on Electrochemical Properties of Choline Chloride Based Eutectic Solvent in Mineral Processing *1OBETEN, ME; UGI, BU; ALOBI, NO Department of Chemical Sciences, Cross River University of Technology, P. M. B. 1123 Calabar - Cross River State, Nigeria. ABSTRACT: Our review highlights the most recent developments in ionic liquid (IL) chemistry where the “well-known” description of IL properties sometimes proves to be inaccurate. However, in the authors’ opinion, all these new research developments concerning ionic liquid properties serve to update knowledge on the typical physical and chemical properties of ILs, which is significant to both theoretical research and industrial applications. Therefore, rather than attempting to give a comprehensive overview of ionic liquid chemistry, the paper presents an opportunity to understand deep eutectic solvents (DES) through a more complete and accurate view . © JASEM https://dx.doi.org/10.4314/jasem.v21i5.29 Keywords : Eutectic; Solvents; Electrochemistry; Ionic Liquids; purification; Ethylene glycol. Since environmental pollution caused by chemical mixed with different hydrogen bond donors (HBDs) and energy industries has increased for several such as urea, ethylene glycol, acetamide or decades, there is high expectation from scientists and hexanediol (type IV DES). engineers to design sustainable chemical processes, to generate less harmful materials and more Owing to its low cost, biodegradability and low environmentally friendly sources of energy toxicity, ChCl was widely used as an organic salt to production. -

Student Number: 201477310
COPYRIGHT AND CITATION CONSIDERATIONS FOR THIS THESIS/ DISSERTATION o Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. o NonCommercial — You may not use the material for commercial purposes. o ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. How to cite this thesis Surname, Initial(s). (2012) Title of the thesis or dissertation. PhD. (Chemistry)/ M.Sc. (Physics)/ M.A. (Philosophy)/M.Com. (Finance) etc. [Unpublished]: University of Johannesburg. Retrieved from: https://ujcontent.uj.ac.za/vital/access/manager/Index?site_name=Research%20Output (Accessed: Date). Metabolomics, Physicochemical Properties and Mycotoxin Reduction of Whole Grain Ting (a Southern African fermented food) Produced via Natural and Lactic acid bacteria (LAB) fermentation A Thesis submitted to the Faculty of Science, University of Johannesburg, South Africa In partial fulfilment of the requirement for the award of a Doctoral Degree in Food Technology By OLUWAFEMI AYODEJI ADEBO STUDENT NUMBER: 201477310 Supervisor : Dr. E. Kayitesi Co-supervisor: Prof. P. B. Njobeh October 2018 EXECUTIVE SUMMARY Drought and challenges related to climate change are some of the issues facing sub-Saharan Africa countries, with dire consequences on agriculture and food security. Due to this prevailing situation, drought and climate resistant crops like sorghum (Sorghum bicolor (L) Moench) can adequately contribute to food security. The versatility and importance of sorghum is well reflected in its use as a major food source for millions of people in sub-Saharan Africa. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl -

Green Chemistry : Greener Alternatives to Synthetic Organic Transformations
Green Chemistry Greener Alternatives to Synthetic Organic Transformations V.K. Ahluwalia Alpha Science International Ltd. Oxford, U.K. Contents Preface v Parti 1. Introduction 1.1-1.10 1.1 Principles of Green Chemistry 1.1 1.2 How to Plan a Green Synthesis 1.2 Part II Green Alternatives to Synthesis Organic Transformations 2. Aqueous Phase Transformations 2.1-2.48 2.1 p-Acetylaminophenol (Tylenol) 2.1 2.2 3-Aminopyridine 2.2 2.2a Anthranilic Acid 2.3 2.3 Benzilic Acid 2.3 2.4 Benzoin 2.5 2.5 Benzotriazole 2.6 2.6 2-Benzoyl-3,5-dimethylbenzofuran 2.7 2.7 n-Butyl Bromide 2.8 2.8 Tert.Butylchloride 2.8 2.9 Chalcone (Benzalacetophenone) 2.9 2.10 Cycloheptanone 2.10 2.11 2,3-Dihydroxy Anisole (Pyrigallol Monomethyl-ether) 2.12 2.12 2,4-Dihydroxybenzoic acid (P-resorcylic Acid) 2.13 2.13 3,4-Dimethoxyphenol 2.14 2.14 2,3-Dimethyl-l-phenylpyrazol-5-one 2.15 2.15 3, 5-Dimethylpyrazole 2.16 2.16 5,5-Diphenylhydantoin 2.17 2.17 Endo-cis-1,4-endoxo-A5-cyclohexene-2,3-dicarboxylic Acid 2.18 2.18 p-Ethoxyacetanilide (Phenacetin) 2.19 2.19 6-Ethoxycarbonyl-3,5-diphenyI-2-cyclohexenone 2.20 2.20 HeteroDiels-AlderAdduct 2.22 2.21 Hippuric Acid (Benzoyl Glycine) 2.22 Viii Contents 2.22 Hydantion 2.23 2.25 3-Hydroxy-3-phenyl-2-methylene Proponamide 2.24 2.24 Iodoform 2.25 2.25 Inodole 2.26 2.26 3-(p-Methoxyphenyl)-2H-1,4-Benzoxazine 2.27 2.27 3-Methylcyclopent-2-enone 2.27 2.28 2-(2'-Methlindol-3yl)-1,4-benzoquinone 2.28 2.29 2-methyl-2-(3-oxobutyl)-1,3-cyclopentanedione 2.29 2.30 P-Naphthyl Acetate 2.30 2.31 (5-Naphthyl Methyl Ether (Nerolin) 2.31 2.32 -

Inhibition of Growth, Synthesis, and Permeability in Neurospora Crassa by Phenethyl Alcohol
JOURNAL OF BACTERIOLOGY, JUly, 1965 Vol. 90, No. I Copyright © 1965 American Society for Microbiology Printed in U.S.A. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol GABRIEL LESTER Department of Biology, Reed College, Portland, Oregon Received for publication 22 January 1965 ABSTRACT LESTER, GABRIEL (Reed College, Portland, Ore.). Inhibition of growth, synthesis, and permeability in Neurospora crassa by phenethyl alcohol. J. Bacteriol. 90: 29-37. 1965.- Inhibition of the growth of Neurospora crassa in still culture was detected at 0.05% and was complete at a level of 0.2% phenethyl alcohol (PEA). Benzyl alcohol was less in- hibitory, and 3-phenyl-1-propanol and phenol were more inhibitory, than PEA; benzyl- amine and phenethylamine were less inhibitory than the analogous hydroxylated com- pounds. Inhibition by PEA was not reversed by synthetic mixtures of purines and pyrimidines or vitamins, or by casein digests, yeast extract, or nutrient broth. The germination of conidia was inhibited by PEA, but after an exposure of 8.5 hr no loss of viability was observed. The addition of PEA to growing shake cultures caused a simul- taneous inhibition of growth and of the syntheses of ribonucleic and deoxyribonucleic acids and protein; the relationships of these compounds to mycelial dry weight and to one another were constant in growing mycelia, and PEA did not significantly affect these relationships. PEA partially inhibited the uptake of glucose, but severely re- stricted the accumulation of L-leucine, L-tryptophan, or a-aminoisobutyric acid in germinated conidia. The efflux of a-aminoisobutyric acid from germinated conidia was somewhat enhanced by PEA, but this effect was not so pronounced as the (complete) inhibition of a-aminoisobutyric acid accumulation by PEA. -

United States Patent (19) 11 4,267,375 Maasbol Et Al
United States Patent (19) 11 4,267,375 Maasbol et al. 45 May 12, 1981 54 PREPARATION OF THIOETHERS OTHER PUBLICATIONS 75) Inventors: Alfred G. Maasbol, Hamburg, Fed. I. Ruderman et al., J. Amer. Chem. Soc., 71, pp. Rep. of Germany; Lothar G. Dulog, 2264-2265, (1949). St. Martens Latem, Belgium Morrisson and Boyd, Organic Chemistry, 2nd edition, (1967), pp. 29–30. 73) Assignee: s.a. Texaco Belgium in.v., Brussels, T. Todsen et al., J. Amer. Chem. Soc., 72, pp. Belgium 4000-4002, (1950). Berichte Deutsch. Chemie, vol. 1, pp. 587-591, (1935), (21) Appl. No.: 945,273 Berlin. 22 Filed: Sep. 25, 1978 D. Gregg et al., J. Org. Chem., pp. 246-252, (1950). M. Malinovskii, Epoxides and Their Derivatives, pp. Related U.S. Application Data 131-136, (1965), Jerusalem, Daniel Davey & Co. Primary Examiner-Glennon H. Hollrah 63 Continuation of Ser. No. 703,045, Jul. 6, 1976, aban Assistant Examiner-M. C. Eakin doned. Attorney, Agent, or Firm-Carl G. Ries; Robert A. 30 Foreign Application Priority Data Kulason; Carl G. Seutter Nov. 19, 1975 GB United Kingdom ............... 47582/75 57 ABSTRACT .. 51 Int. Cl. ............................................ CO7C 149/30 Thioethers may be prepared by reacting a thiol, such as thiophenol, with an alcohol (having electron donor 52 U.S. C. ......................................... 568/57; 568/58 groups in the alpha or beta position to its hydroxyl 58 Field of Search ........................ 260/609 E, 609 R group) such as phenyl-1-hydroxy-phenethylsulfide. Re 56) References Cited action is carried out in the presence of a Lewis Acid U.S. PATENT DOCUMENTS metal halide, typically zinc chloride. -
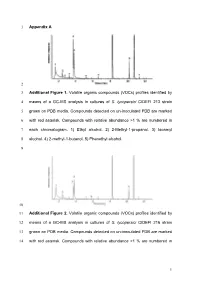
Appendix a 1 2 Additional Figure 1. Volatile Organic Compounds (Vocs
1 Appendix A 2 3 Additional Figure 1. Volatile organic compounds (VOCs) profiles identified by 4 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 213 strain 5 grown on PDB media. Compounds detected on un-inoculated PDB are marked 6 with red asterisk. Compounds with relative abundance >1 % are numbered in 7 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 8 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 9 10 11 Additional Figure 2. Volatile organic compounds (VOCs) profiles identified by 12 means of a GC-MS analysis in cultures of S. lycopersici CIDEFI 216 strain 13 grown on PDB media. Compounds detected on un-inoculated PDB are marked 14 with red asterisk. Compounds with relative abundance >1 % are numbered in 1 15 each chromatogram. 1) Ethyl alcohol. 2) 2-Methyl-1-propanol. 3) Isoamyl 16 alcohol. 4) 2-methyl-1-butanol. 5) Phenethyl alcohol. 6) Furfuryl alcohol. 17 18 19 Additional Figure 3. Volatile organic compounds (VOCs) profiles identified by 20 means of a GC-MS analysis in cultures of F. fulva CIDEFI 300 strain grown on 21 PDB media. Compounds detected on un-inoculated PDB are marked with red 22 asterisk. Compounds with relative abundance >1 % are numbered in each 23 chromatogram. 6) Furfuryl alcohol. 7) Acetone. 8) Methyl trimethylacetate. 9) 24 Isoamyl alcohol. 10) 1-Octene. 11) 3-Hexanone, 4-methyl-. 12) Styrene. 13) 3- 25 Octanone. 14) Hexanoic acid, 2 ethyl-, methyl ester. 15) 2-Nonanone. 16) 26 Phenethyl alcohol. 17) No identified Nist05. 27 2 28 29 Additional Figure 4. -

Chemicals That Form Explosive Levels of Peroxides Without Concentration (Safe Storage Time After Opening - 3 Months) Chemical CAS # Synonym State Ref
Group A- Chemicals that form explosive levels of peroxides without concentration (Safe storage time after opening - 3 months) Chemical CAS # Synonym State Ref. 000106- Butadiene(1,3) 1,3-Butadiene gas 4 99-0 000126- 2-Chloro-1,3- Chloroprene (1,3) liquid 4 99-8 butadiene 000821- Divinyl acetylene 1,5-Hexadien- 3-yne liquid 5 08-9 000108- Isopropyl ether liquid 5 20-3 000116- Tetrafluoroethylene gas 4 14-3 000109- Vinyl ether Divinyl ether liquid 5 93-3 000075- 1,1- Vinylidene chloride liquid 5 35-4 Dichloroethylene Group B-Chemicals that form explosive levels of peroxides on concentration (Safe storage time after opening - 12 months) Chemical CAS # Synonym State Ref. 000105- Acetal liquid 5 57-7 000075- Acetaldehyde liquid 4 07-0 000100- Benzyl alcohol liquid 4 51-6 000078- 2-Butanol liquid 4 92-2 000108- Cyclohexanol liquid 4 93-0 000110- Cyclohexene liquid 5 83-8 000822- 2-Cyclohexen-1-ol liquid 4 67-3 000142- Cyclopentene liquid 5 29-0 000091- Decahydronaphthalene liquid 4 17-8 000460- Diacetylene gas 5 12-8 000077- Dicyclopentadiene liquid 5 73-6 Diethylene glycol 000111- Diglyme liquid 5 dimethyl ether 96-6 000123- Dioxane 1,4-Dioxane liquid 5 91-1 Ethylene glycol 000110- Glyme liquid 5 dimethyl ether 71-4 000060- Ethyl ether Diethyl ether liquid 5 29-7 000110- Furan liquid 5 00-9 000589- 4-Heptanol liquid 4 55-9 000626- 2-Hexanol liquid 4 93-7 000098- Isopropyl benzene Cumene liquid 5 82-8 000074- Methyl acetylene Propyne gas 5 99-7 000123- 3-Methyl-1-butanol Isoamyl alcohol liquid 4 51-3 000096- Methyl cyclopentane liquid 5 37-7 -

EZ Speed Strip Safety Data Sheet
Date Printed: 10/14/2020 Page 1 / 6 Safety Data Sheet 1. Identification Product Name: EZ Speed Strip Revision Date: 10/14/2020 Product Identifier: 11212500 Recommended Use: Paint Remover/Strip Supplier: Emergency Telephone: Chemtrec: +1-800-424-9300 (USA) Kop-Coat Marine Group Chemtrec: +1 703-527-3887 (Intl.) 36 Pine Street 24 hrs./day, 7 days/week Rockaway, NJ, 07866, USA Poison Control: 1-800-222-1222 1-800-221-4466 (non - emergency matters) 24 Hour Hotline: 847-367-7700 2. Hazard Identification Classification Symbol(s) of Product Signal Word Danger Possible Hazards 87% of the mixture consists of ingredient(s) of unknown acute toxicity. GHS HAZARD STATEMENTS Acute Toxicity, Inhalation, category 4 H332 Harmful if inhaled. Eye Irritation, category 2A H319 Causes serious eye irritation. Flammable Liquid, category 2 H225 Highly flammable liquid and vapour. STOT, single exposure, category 3, NE H336 May cause drowsiness or dizziness. GHS LABEL PRECAUTIONARY STATEMENTS P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P261 Avoid breathing dust/fume/gas/mist/vapors/spray. P264 Wash hands thoroughly after handling. P271 Use only outdoors or in a well-ventilated area. P280 Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353 IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/ shower. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.