Carpinus Orientalis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Demirköy, Kırklareli) Turkey
Işın Z. Ursavaş S. 2018. Anatolian Bryol……………………………………………………………….92 Anatolian Bryology http://dergipark.gov.tr/anatolianbryology Anadolu Briyoloji Dergisi Research Article DOI: 10.26672/anatolianbryology.472405 e-ISSN:2458-8474 Online The Moss Flora of İğneada Floodplain Forests National Park (Demirköy, Kırklareli) Turkey Zeki IŞIN (Orcid: 0000-0002-7637-061X)1, *Serhat URSAVAŞ (Orcid: 0000-0001-5480-5590)2 1Department of Forest Engineering, Graduate School of Natural and Applied Sciences, Çankırı Karatekin University, 18200, Çankırı, TURKEY; 2Department of Forest Engineering, Faculty of Forestry, Çankırı Karatekin University, 18200, Çankırı, TURKEY Received: 19.10.2018 Revised: 01.11.2018 Accepted: 30.11.2018 Abstract In this study, the moss flora of İğneada Floodplain Forest National Park (Kırklareli-Demirköy) in Turkey were investigated between the years of 2015-2016. As a result of examination of six hundred thirty moss samples, which collected from İğneada Floodplain Forest National Park, were examined 24 families, 55 genera, 102 taxa species or subspecies. In terms of taxa number, the richest six families are; Pottiaceae (20), Brachytheciaceae (14), Polytrichaceae (9), Orthotrichaceae (8), Hypnaceae (6), Bryaceae (6). Atrichum crispum (James) Sull., and Bryum gemmiferum (R. Wilczek & Demaret.) (in press), marked with a black diamond (♦) sign are new records for the Turkish bryophyte flora. According to Henderson (1961) grid square system, 17 moss taxa marked with an asterisk (*) sing are new records for A1 square. While acrocarpous taxa (70) represent 68 % of the whole flora, the ratio of pleurocarpous ones (32) is 32 %. Key words: Atrichum crispum, Bryum gemmiferum, new record, Kırklareli-Demirköy, national park, moss, flora, Turkey İğneada Longoz Ormanları Milli Parkı (Demirköy, Kırklareli) Karayosunu Florası Öz Bu çalışmada, 2015-2016 yılları arasında İğneada Longoz Ormanları, Milliparkında (Kırklareli- Demirköy) alanın karayosunu florası araştırılmıştır. -

Global Survey of Ex Situ Betulaceae Collections Global Survey of Ex Situ Betulaceae Collections
Global Survey of Ex situ Betulaceae Collections Global Survey of Ex situ Betulaceae Collections By Emily Beech, Kirsty Shaw and Meirion Jones June 2015 Recommended citation: Beech, E., Shaw, K., & Jones, M. 2015. Global Survey of Ex situ Betulaceae Collections. BGCI. Acknowledgements BGCI gratefully acknowledges the many botanic gardens around the world that have contributed data to this survey (a full list of contributing gardens is provided in Annex 2). BGCI would also like to acknowledge the assistance of the following organisations in the promotion of the survey and the collection of data, including the Royal Botanic Gardens Edinburgh, Yorkshire Arboretum, University of Liverpool Ness Botanic Gardens, and Stone Lane Gardens & Arboretum (U.K.), and the Morton Arboretum (U.S.A). We would also like to thank contributors to The Red List of Betulaceae, which was a precursor to this ex situ survey. BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) BGCI is a membership organization linking botanic gardens is over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. www.bgci.org FAUNA & FLORA INTERNATIONAL (FFI) FFI, founded in 1903 and the world’s oldest international conservation organization, acts to conserve threatened species and ecosystems worldwide, choosing solutions that are sustainable, based on sound science and take account of human needs. www.fauna-flora.org GLOBAL TREES CAMPAIGN (GTC) GTC is undertaken through a partnership between BGCI and FFI, working with a wide range of other organisations around the world, to save the world’s most threated trees and the habitats which they grow through the provision of information, delivery of conservation action and support for sustainable use. -

Field Guide – Common Trees and Shrubs of Georgia
Introduction Up to 400 species of trees and shrubs grow in Georgian for- ests. This Field Guide contains information about 100 species of trees and shrubs from 38 plant families. The abundance of relict and endemic timber species (61 species endemic to Geor- gia and 43 species endemic to the Caucasus) indicates the high biodiversity of Georgian forests. Georgian forests provide habitats and migration corridors to a range of wild fauna, and play an important role in the conserva- tion of the genetic diversity of animal species in the region. In conditions of complex and deeply dissected relief, characteristic to Georgia, forests are especially important due to their climate regulation, water regulation and soil protection functions. Forests also ensure the continuous delivery of vital benefits and resources to the population, and facilitate the development of a range of industries. Introduction In this Field Guide each plant family is displayed in a different color. The Field Guide contains an alphabetical index of species, as well as the names of species in Latin and English, as estab- lished by the International Code of Botanical Nomenclature. The Field Guide also contains a brief description of the taxo- nomic characteristics, range and protection status of each spe- cies. Alphabetical Index Name in English Name in Latin # Alpine Currant Ribes alpinum 59 Bay Laurel Laurus nobilis 62 Begonia-Leafed Lime Tilia Begoniifolia 92 Bitchvinta Pine Pinus pithyusa 6 Black Alder Alnus barbata 28 Black Elder Sambucus nigra 31 Black Poplar Populus -

Carpinus L. Hornbeam Or Ironwood
C Betulaceae—Birch family Carpinus L. hornbeam or ironwood Paula M. Pijut Dr. Pijut is a plant physiologist at the USDA Forest Service’s North Central Research Station, Hardwood Tree Improvement and Regeneration Center,West Lafayette, Indiana Growth habit, occurrence, and use. The hornbeam Appalachian Mountains and northern interior regions to the genus—Carpinus L.—includes about 35 species of decidu- West (Furlow 1987b). The Latin American C. tropicalis is ous, monoecious, small to large trees, that are native to the divided into subsp. tropicalis of the highlands of southern Northern Hemisphere from Europe to eastern Asia, south to Mexico and north Central America and subsp. mexicana of the Himalayas, and in North and Central America (Furlow the mountains in northeastern Mexico and the trans-Mexican 1990; Hillier 1991; Krüssmann 1984; LHBH 1976; Suszka volcanic belt (Furlow 1987b). and others 1996). Five species are considered here (table The wood of hornbeams is extremely hard—hence the 1). Hornbeams occur mainly as understory trees in rich, common name “ironwood”—and is used for making tool moist soils on bottomlands and on protected slopes handles and mallet heads. It is also used to produce the (Metzger 1990; Rudolf and Phipps 1974). European horn- high-quality charcoal used in gunpowder manufacture beam is an important forest tree species throughout Europe (Bugala 1993; Furlow 1990). Species of ornamental interest (Furlow 1990). In Mexico and Central America, Carpinus in the United States are listed in table 1. Most of the infor- tropicalis (J.D. Sm.) Lundell forms a dominant canopy mation presented in this chapter deals with European and component (Furlow 1990). -

Ostrya Carpinifoliacarpinifolia Hop-Hornbeam,Hop-Hornbeam, Hophornbeamhophornbeam
OstryaOstrya carpinifoliacarpinifolia hop-hornbeam,hop-hornbeam, hophornbeamhophornbeam Ostrya carpinifolia (Hop-hornbeam) naturally occurs in South Europe and in Asia Minor. The hop-hornbeam grows in the southernmost regions in moist environments with partial shade; in the northern regions, the tree acts more as a pioneer species and grows in light, warm and underdeveloped soil. Ostrya carpinifolia grows in thin, rocky soil and forms the undergrowth in the Pinus nigraforest, along with other species including Carpinus orientalis,Fraxinus ornusand Quercus pubescens. In its original distribution area, the hop-hornbeam grows up to 25 metres tall; in cultivation, it usually does not grow to be more than 18 metres tall with a wide, irregular wide egg-shaped to round crown up to 15 metres wide. Ostrya carpinifoliais a host plant for white truffles. The hop-hornbeam blooms yellow-green in April with both striking male and short female catkins. In summer, its fruits are even more striking than its blooms: nuts in large, white-green, hop-like fruits and - turning brown - stay on the tree until deep into autumn. The egg-shaped, doubly serrate leaves are dark green, with a light green, hairy underside and turn yellow in autumn. Both the leaves and the tree shape of the hop-hornbeam are similar to those of Carpinus.At an early age, Ostrya carpinifoliahas bark covered in lenticels; when it is older, the bark becomes rough and peels off. Ostrya carpinifolia is mainly suitable for use in parks, central reservations and gardens. But the tree can also be used in wide streets and avenues, as it tolerates pavement and drought quite well. -

(Carpinus Turczaninovii) Based on Nuclear Ribosomal ITS Sequence
African Journal of Biotechnology Vol. 10(76), pp. 17435-17442, 30 November, 2011 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.1337 ISSN 1684–5315 © 2011 Academic Journals Full Length Research Paper Phylogeny of Korean Hornbeam ( Carpinus turczaninovii ) based on nuclear ribosomal ITS sequence Sun, Y. L. 1, Wang, D. 1, Lee, H. B.2, Park, W. G. 2, Kwon, O. W. 3 and Hong, S. K.1,4 * 1Department of Bio-Health Technology, Kangwon National University, Chuncheon, Kangwon-Do, 200-701, Korea. 2Department of Forest Resources, Kangwon National University, Chuncheon, Kangwon-Do, 200-701, Korea. 3Korea Forest Seed and Variety Center, Suanbo, Chungju, Chungcheongbuk-Do, 380-941, Korea. 4Division of Biomedical Technology, College of Biomedical Science, Chuncheon, Kangwon-Do, 200-701, Korea. Accepted 25 July, 2011 The genus Carpinus belonging to Coryloideae, Betulaceae, has significant economic and ornamental importance. This study was undertaken with the aim to understand the genetic diversity among eighteen isolates of Carpinus turczaninovii collected from different geographical regions of Korea, using ribosomal RNA (rRNA) internal transcribed spacer (ITS) sequences, to compare the infraspecific- phylogenetic relationships among C. turczaninovii in Korea, and some known Carpinus plants. The size variation of sequenced rRNA ITS regions was not seen, with 215, 162, 222 bp of ITS1 region, 5.8S rRNA gene, ITS2 region, respectively. However, some certain nucleotide variations resulted in genetic diversity. In the genus Carpinus , C. turczaninovii closely genetic with Castanopsis kawakamii , Carpinus orientalis , Carpinus monbeigiana , and Calyptranthes polynenra formed one monophyletic clade, while Carpinus betulus and Carpinus laxiflora, respectively formed one monophyletic clade. -

Oak & Oak Forests in Caucasia
OAIQ Al'D OAK FORESTS I CAL CASL\ Peter A. chmidt Profc,.;er, Dre..Oen CnJ\eNt~ of Technology Depanment ot Fore't Sc1ence,, ln,otute of General Ecolog) and FnHronment \fanagement D-01737 Tharandt. German) Introduction to the Cauca! lb ReWoo When reganlmg the .. Caucasu~:· some authol"> reh:r onl) to the h1gh mountam~ of the Great Caucasu\. Here the term "Cauca,iu'' '' 1ll be: u'ed to reler to the rnure area located bet\\.een the Black Sea and the Ca..,p1an Sea nl the Cauca'u' Reg1on . Be..,1de~ the Great Cauca~u~. the Nonh Cau~.:<hiUn Plain belonging to the Ru\\lan Federation, ~well ,L\ the depre\siOm. and lnwlanth. muumam rangcs and the h1ghland south of the Great Cauca~u' up tn t11e 'outhem border of the ..,t,•le' Armema. AserbaiJan. Georg1a <South Cauca,ta, often called ''Transcaucasia l are abo included. The Great Cauca'u' extend~ betv.een the Black Sea and Casp1an Sea o\er a t.l1..,tan~.:c ul I 500 km and a breadth of 30-180 km. It wn'1't' of sc\ eml parallel mountain nrnges. The mam ndge i~ preceded toward' the nonh by a 'crond ndge (here the Elbrus, the highe't peak 5633 m. and the Ka,bel..:. the h1ghN peak 5C.I-W ml. a~ well a' the rock) ndge Cch3Jll of bme..tone mas 1h). The e h1gh mountam' form an 1mpo1U11t chmate and "ater di' ide bet\\ een Ea't Europe and South\\e't Asia, and thu' hetween t\\O continent'. -

A Critical Taxonomic Checklist of Carpinus and Ostrya (Coryloideae, Betulaceae)
European Journal of Taxonomy 375: 1–52 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.375 www.europeanjournaloftaxonomy.eu 2017 · Holstein N. & Weigend M. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph No taxon left behind? – a critical taxonomic checklist of Carpinus and Ostrya (Coryloideae, Betulaceae) Norbert HOLSTEIN 1,* & Maximilian WEIGEND 2 1,2 Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Nordrhein-Westfalen, Germany. * Corresponding author: [email protected] 2 Email: [email protected] Abstract. Hornbeams (Carpinus) and hop-hornbeams (Ostrya) are trees or large shrubs from the northern hemisphere. Currently, 43 species of Carpinus (58 taxa including subdivisions) and 8 species of Ostrya (9 taxa including sudivisions) are recognized. These are based on 175 (plus 16 Latin basionyms of cultivars) and 21 legitimate basionyms, respectively. We present an updated checklist with publication details and type information for all accepted names and the vast majority of synonyms of Carpinus and Ostrya, including the designation of 54 lectotypes and two neotypes. Cultivars are listed if validly described under the rules of the ICN. Furthermore, we consider Carpinus hwai Hu & W.C.Cheng to be a synonym of Carpinus fargesiana var. ovalifolia (H.J.P.Winkl.) Holstein & Weigend comb. nov. During the course of our work, we found 30 legitimate basionyms of non-cultivars that have been consistently overlooked since their original descriptions, when compared with the latest checklists and fl oristic treatments. As regional fl oras are highly important for taxonomic practice, we investigated the number of overlooked names and found that 78 basionyms were omitted at least once in the eight regional treatments surveyed. -

Comparative Anatomy of Carpinus Orientalis Mill. (Betulaceae) Populations in Iran
Journal of Genetic Resources J Genet Resour 2015;1(1):45-54 http://sc.journals.umz.ac.ir doi:10.22080/jgr.2015.1123 University of Mazandaran Iranian Biology Society Comparative Anatomy of Carpinus orientalis Mill. (Betulaceae) Populations in Iran Mahsa Razaz1, Alireza Naqinezhad*1, Arman Mahmoudi Otaghvari1, Abasalt Hosseinzadeh Colagar2 and Rouhangiz Abbas Azimi3 1Department of Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran. 2Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran. 3Departmnet of Botany, Research Institute of Forests and Rangelands, Tehran, Iran *Corresponding author: [email protected] Received: 12 August 2013 Accepted: 10 January 2014 Abstract Natural populations of Carpinus orientalis Mill. shrublands occur mainly in high and middle altitudes of the Hyrcanian forests, N. Iran, particularly on steep rocks and forest outcrops. There are some discrepancies on the intra-specific delimitation of this important woody species. The aim of the current study is to examine the anatomical variation of stems and leaves of sixteen populations of Carpinus orientalis collected in four north and northeastern provinces of Iran for the first time. It was shown that the anatomical characteristics of stem have some correlations with climatic variables such as temperature, altitude and precipitation, whereas midrib anatomical characteristics did not show any correlation with the above mentioned parameters. Key words: Anatomical characters; Carpinus orientalis; Hyrcanian forest; Climatic factors Introduction Salsola kali subsp. ruthenica (Chenopodiaceae) proved that this species is well adapted to the The genus Carpinus L. belongs to Betulaceae harsh conditions (Bercu & Bavaru, 2004). Stem family (Yoo and Wen, 2002, Yoo and Wen, 2007; xylem features in two evergreen Quercus species Chang, 2004); a well-defined family with six and a deciduous one were analyzed along an genera and 130 species (Chen, 1994). -

New and Rare Ornamental Woody Plants Recently Distributed by The
ARNOLDIA A continuation of the BULLETIN OF POPULAR INFORMATION of the Arnold Arboretum, Harvard University VOLUME 16 OCTOBER 12, I9~6 NUMBERS 7-9 NEW AND RARE ORNAMENTAL WOODY PLANTS RECENTLY DISTRIBUTED BY THE ARNOLD ARBORETUM of the functions of the Arnold Arboretum always has been to distribute ONEnew or rare ornamentals of high quality to the commercial propagators and so make them available to the plant-buying public. Many gardeners in the north- ern United States may not have had the opportunity to realize that the Arnold Arboretum has been doing this since it was established in 18 i 2. Propagating material of plants not available in the trade in the form of seeds, cuttings, scions and budwood is frequently given commercial nurserymen who request specific items. Fifteen years ago a special program for the distribution of new or rare plants was started, and over a hundred species and varieties of new or rare orna- mental woody plants have been distributed to commercial sources as a result. It is always easy to lose sight of the fact that new plants are being made available ’ to commercial sources (especially when no extensive advertising accompanies the program !). In 19L1 a general program of propagation was started at the Arnold Arboretum in which particular new or rare ornamental woody plants were specifi- cally grown for the nurseryman. Our experience had shown (see ARNOLDIA, Series 4, Vol. VIII, No. 3, May 1940) that seeds, buds, cuttings and scions when given to nurserymen frequently "failed" for several reasons. As a result, rare specimens in the Arboretum were being heavily cut in order to provide commercial growers with propagating ma- terial. -
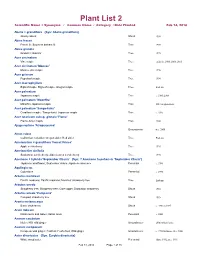
Plants at Homes Database
Plant List 2 Scientific Name + Synonyms / Common Name / Category / Date Planted Feb 14, 2016 Abelia × grandiflora {Syn: Abelia grandiflora} Glossy abelia Shrub 1994 Abies fraseri Fraser fir; Southern balsam fir Tree 1998 Abies grandis Grand fir; Giant fir Tree 1995 Acer circinatum Vine maple Tree endemic; 2000; 2008; 2013 Acer circinatum 'Monroe' Monroe vine maple Tree 1996 Acer griseum Paperbark maple Tree 1994 Acer macrophyllum Bigleaf maple; Big leaf maple; Oregon maple Tree Endemic Acer palmatum Japanese maple Tree c. 1985; 2008 Acer palmatum 'Moonfire' Moonfire Japanese maple Tree 2013 to open areas Acer palmatum 'Sango-kaku' Coral-bark maple; 'Sango-kaku' Japanese maple Tree c. 1995 Acer tataricum subsp. ginnala 'Flame' Flame Amur maple Tree 2000 Ajuga reptans 'Atropurpurea' Groundcover tx c. 2000 Alnus rubra Californian red alder; Oregon alder; Red alder Tree Endemic Amelanchier × grandiflora 'Forest Prince' Apple serviceberry Tree 1995 Amelanchier alnifolia Saskatoon serviceberry; Alder-leaved serviceberry Tree 1995 Anemone × hybrida 'September Charm' {Syn: ? Anemone hupehensis 'September Charm'} Japanese windflower; September charm Japanese anemone Perennial c. 1988 Aquilegia sp. Columbine Perennial c. 1995 Arbutus menziesii Pacific madrone; Pacific madrona; Menzies' strawberry tree Tree Endemic Arbutus unedo Strawberry tree; Strawberry tree; Cane apple; Dalmatian strawberry Shrub 1984 Arbutus unedo 'Compacta' Compact strawberry tree Shrub 1995 Aronia melanocarpa Black chokeberry Shrub c. 1990; tx 1995 Arum italicum Italian lords and ladies; Italian arum Perennial c. 2000 Asarum caudatum Native NW wild ginger Groundcover 2006 tx from home Asarum europaeum European wild ginger; Foalfoot; Foal's foot; Wild ginger Groundcover c. 1990 for Home; tx c. 2000 Aster divaricatus {Syn: Eurybia divaricata} White wood aster Perennial Home 1992, tx c. -

Supplementary Material
Xiang et al., Page S1 Supporting Information Fig. S1. Examples of the diversity of diaspore shapes in Fagales. Fig. S2. Cladogram of Fagales obtained from the 5-marker data set. Fig. S3. Chronogram of Fagales obtained from analysis of the 5-marker data set in BEAST. Fig. S4. Time scale of major fagalean divergence events during the past 105 Ma. Fig. S5. Confidence intervals of expected clade diversity (log scale) according to age of stem group. Fig. S6. Evolution of diaspores types in Fagales with BiSSE model. Fig. S7. Evolution of diaspores types in Fagales with Mk1 model. Fig. S8. Evolution of dispersal modes in Fagales with MuSSE model. Fig. S9. Evolution of dispersal modes in Fagales with Mk1 model. Fig. S10. Reconstruction of pollination syndromes in Fagales with BiSSE model. Fig. S11. Reconstruction of pollination syndromes in Fagales with Mk1 model. Fig. S12. Reconstruction of habitat shifts in Fagales with MuSSE model. Fig. S13. Reconstruction of habitat shifts in Fagales with Mk1 model. Fig. S14. Stratigraphy of fossil fagalean genera. Table S1 Genera of Fagales indicating the number of recognized and sampled species, nut sizes, habits, pollination modes, and geographic distributions. Table S2 List of taxa included in this study, sources of plant material, and GenBank accession numbers. Table S3 Primers used for amplification and sequencing in this study. Table S4 Fossil age constraints utilized in this study of Fagales diversification. Table S5 Fossil fruits reviewed in this study. Xiang et al., Page S2 Table S6 Statistics from the analyses of the various data sets. Table S7 Estimated ages for all families and genera of Fagales using BEAST.