Ecology of the Snow Leopard and the Himalayan Tahr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bushmeat Hunting and Extinction Risk to the World’S Rsos.Royalsocietypublishing.Org Mammals
Downloaded from http://rsos.royalsocietypublishing.org/ on October 26, 2017 Bushmeat hunting and extinction risk to the world’s rsos.royalsocietypublishing.org mammals 1,2 4,5 Research William J. Ripple , Katharine Abernethy , Matthew G. Betts1,2, Guillaume Chapron6, Cite this article: Ripple WJ et al.2016 Bushmeat hunting and extinction risk to the Rodolfo Dirzo7, Mauro Galetti8,9, Taal Levi1,2,3, world’s mammals R. Soc. open sci. 3: 160498. 10,11 12 http://dx.doi.org/10.1098/rsos.160498 Peter A. Lindsey , David W. Macdonald , Brian Machovina13, Thomas M. Newsome1,14,15,16, Carlos A. Peres17, Arian D. Wallach18, Received: 10 July 2016 Accepted: 20 September 2016 Christopher Wolf1,2 and Hillary Young19 1GlobalTrophic Cascades Program, Department of Forest Ecosystems and Society, 2Forest Biodiversity Research Network, Department of Forest Ecosystems and Society, and 3Department of Fisheries and Wildlife, Oregon State University, Corvallis, Subject Category: OR 97331, USA Biology (whole organism) 4School of Natural Sciences, University of Stirling, Stirling FK9 4LA, UK 5Institut de Recherche en Ecologie Tropicale, CENAREST, BP 842 Libreville, Gabon Subject Areas: 6Grimsö Wildlife Research Station, Department of Ecology, Swedish University of ecology Agricultural Sciences, 73091 Riddarhyttan, Sweden 7Department of Biology, Stanford University, Stanford, CA 94305, USA Keywords: 8Universidade Estadual Paulista (UNESP), Instituto Biociências, Departamento de wild meat, bushmeat, hunting, mammals, Ecologia, 13506-900 Rio Claro, São Paulo, Brazil extinction 9Department of Bioscience, Ecoinformatics and Biodiversity, Aarhus University, 8000 Aarhus, Denmark 10Panthera, 8 West 40th Street, 18th Floor, New York, NY 10018, USA 11 Author for correspondence: Mammal Research Institute, Department of Zoology and Entomology, University of William J. -

Tibet's Biodiversity
Published in (Pages 40-46): Tibet’s Biodiversity: Conservation and Management. Proceedings of a Conference, August 30-September 4, 1998. Edited by Wu Ning, D. Miller, Lhu Zhu and J. Springer. Tibet Forestry Department and World Wide Fund for Nature. China Forestry Publishing House. 188 pages. People-Wildlife Conflict Management in the Qomolangma Nature Preserve, Tibet. By Rodney Jackson, Senior Associate for Ecology and Biodiversity Conservation, The Mountain Institute, Franklin, West Virginia And Conservation Director, International Snow Leopard Trust, Seattle, Washington Presented at: Tibet’s Biodiversity: Conservation and Management. An International Workshop, Lhasa, August 30 - September 4, 1998. 1. INTRODUCTION Established in March 1989, the Qomolangma Nature Preserve (QNP) occupies 33,819 square kilometers around the world’s highest peak, Mt. Everest known locally as Chomolangma. QNP is located at the junction of the Palaearctic and IndoMalayan biogeographic realms, dominated by Tibetan Plateau and Himalayan Highland ecoregions. Species diversity is greatly enhanced by the extreme elevational range and topographic variation related to four major river valleys which cut through the Himalaya south into Nepal. Climatic conditions differ greatly from south to north as well as in an east to west direction, due to the combined effect of exposure to the monsoon and mountain-induced rain s- hadowing. Thus, southerly slopes are moist and warm while northerly slopes are cold and arid. Li Bosheng (1994) reported on biological zonation and species richness within the QNP. Surveys since the 1970's highlight its role as China’s only in-situ repository of central Himalayan ecosystems and species with Indian subcontinent affinities. Most significant are the temperate coniferous and mixed broad-leaved forests with their associated fauna that occupy the deep gorges of the Pungchu, Rongshar, Nyalam (Bhote Kosi) and Kyirong (Jilong) rivers. -

Arabian Tahr in Oman Paul Munton
Arabian Tahr in Oman Paul Munton Arabian tahr are confined to Oman, with a population of under 2000. Unlike other tahr species, which depend on grass, Arabian tahr require also fruits, seeds and young shoots. The areas where these can be found in this arid country are on certain north-facing mountain slopes with a higher rainfall, and it is there that reserves to protect this tahr must be made. The author spent two years in Oman studying the tahr. The Arabian tahr Hemitragus jayakari today survives only in the mountains of northern Oman. A goat-like animal, it is one of only three surviving species of a once widespread genus; the other two are the Himalayan and Nilgiri tahrs, H. jemlahicus and H. hylocrius. In recent years the government of the Sultanate of Oman has shown great interest in the country's wildlife, and much conservation work has been done. From April 1976 to April 1978 I was engaged jointly by the Government, WWF and IUCN on a field study of the tahr's ecology, and in January 1979 made recommendations for its conservation, which were presented to the Government. Arabian tahr differ from the other tahrs in that they feed selectively on fruits, seeds and young shoots as well as grass. Their optimum habitat is found on the north-facing slopes of the higher mountain ranges of northern Oman, where they use all altitudes between sea level and 2000 metres. But they prefer the zone between 1000 and 1800m where the vegetation is especially diverse, due to the special climate of these north-facing slopes, with their higher rainfall, cooler temperatures, and greater shelter from the sun than in the drought conditions that are otherwise typical of this arid zone. -
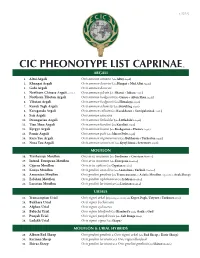
Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26. -

The Role of Sherpa Culture in Nature Conservation
The Role of SHERPA CULTURE in NATURE CONSERVATION Copyright © Khumbu Sherpa Culture Conservation Society www.khumbusherpaculture.org Book : The Role of Sherpa Culture in Nature Conservation Publisher : Khumbu Sherpa Culture Conservation Society (KSCCS) Published Year : 2073 B.S. Edition : First Writer & Photographer : Tenzing Tashi Sherpa Typing & Translation : Tsherin Ongmu Sherpa Editor : Professor Stan Stevens, Ph.D. Design, Layout & Print : Digiscan Pre-press Pvt. Ltd., Naxal, Kathmandu The Role of SHERPA CULTURE in NATURE CONSERVATION Table of Contents 1. The Role of Sherpa Culture in Nature Conservation 1 Khumbu is a Sherpa Community Conserved Area 2 Sacred Himalayas 3 Sacred Lakes - Gokyo Lake 5 Springs 9 Religious Conserved Forests 10 Community Conserved Forest 11 Bird Conservation Area 12 Grazing Management Areas for Livestock 12 Conservation Tradition 13 Nawa System for Conservation 14 The Rules of Singhki Nawa (Wood Custodian) 14 The Custom of the Lhothok Nawa (Crop and Pastures Custodian) 15 The Work and the Duty Term of the Nawa and Worshyo 17 Yulthim (Community Assembly) 18 The Rules and Laws of Community 19 Short Story by Reincarnated Lama Ngawang Tenzing Zangbu about Nawa 20 The Sacred Worship Areas of Sherpas 21 Nangajong 21 Worshyo 22 Pangboche 23 Places in Between Fungi Thyanga Bridge and Pangboche Bridge 25 Khumjung and Khunde 29 Khumbu’s Chortens 33 Agriculture of Khumbu 35 Mountains Around Khumbu 38 2. The Role of KSCCS in Nature Conservation 39 A. Cultural Interaction 39 B. Cultural and ICCA Educational Tour 40 1. Community Tour 40 2. Sherpa Culture and Conservation Tour for Students Organized by Khumjung by KSCCS 41 3. -

Life History Account for Himalayan Tahr
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group HIMALAYAN TAHR Hemitragus jemlahicus Family: BOVIDAE Order: ARTIODACTYLA Class: MAMMALIA M185 Written by: R. A. Hopkins Reviewed by: H. Shellhammer Edited by: J. Harris, S. Granholm DISTRIBUTION, ABUNDANCE, AND SEASONALITY The tahr is an uncommon, yearlong resident of valley foothill hardwood and open grassland habitats on the Hearst Ranch, San Luis Obispo Co. (Barrett 1966). Probably no more than a few hundred of these introduced, goat-like animals live on the ranch. Native to Himalayan region, from Kashmir to Sikkim. SPECIFIC HABITAT REQUIREMENTS Feeding: Barrett (1966) suggested that tahr on the Hearst Ranch fed primarily on grasses, forbs, and to a lesser extent on browse, such as live oak, toyon, poison-oak, and laurel. Detailed food habits studies are lacking. Cover: Rock outcrops and cliffs appear to be almost essential for escape cover and for bedding. The tahr, which evolved in a cooler climate, may require shaded woodlands and north-facing slopes in summer. Reproduction: Rock outcrops and rugged cliffs offer protection from predators during breeding. Water: No data found. Pattern: Tahr use a mixture of valley foothill hardwoods and open grasslands, interspersed with rocky outcrops for protection. In native Himalaya habitat, rocky, wooded mountain slopes and rugged hills are preferred (Nowak and Paradiso 1983). SPECIES LIFE HISTORY Activity Patterns: Active yearlong; primarily diurnal. Seasonal Movements/Migration: Non-migratory in areas of moderate topographic relief, such as the Hearst Ranch. Home Range: Bachelor herds of different sizes and age-classes are found, as well as composite bands of mature females, immature bulls, and kids (Anderson and Henderson 1961). -

Himalayan Glacier Mass Changes Altherr, W
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | The Cryosphere Discuss., 4, 2593–2613, 2010 The Cryosphere www.the-cryosphere-discuss.net/4/2593/2010/ Discussions TCD doi:10.5194/tcd-4-2593-2010 4, 2593–2613, 2010 © Author(s) 2010. CC Attribution 3.0 License. Himalayan glacier This discussion paper is/has been under review for the journal The Cryosphere (TC). mass changes Please refer to the corresponding final paper in TC if available. T. Bolch et al. Longest time series of glacier mass Title Page changes in the Himalaya based on stereo Abstract Introduction imagery Conclusions References Tables Figures T. Bolch1,3, T. Pieczonka1, and D. I. Benn2,4 1Institut fur¨ Kartographie, Technische Universitat¨ Dresden, Germany J I 2 The University Centre in Svalbard, Norway J I 3Geographisches Institut, Universitat¨ Zurich,¨ Switzerland 4University of St Andrews, UK Back Close Received: 1 December 2010 – Accepted: 9 December 2010 – Published: 20 December 2010 Full Screen / Esc Correspondence to: T. Bolch ([email protected]) Printer-friendly Version Published by Copernicus Publications on behalf of the European Geosciences Union. Interactive Discussion 2593 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract TCD Mass loss of Himalayan glaciers has wide-ranging consequences such as declining water resources, sea level rise and an increasing risk of glacial lake outburst floods 4, 2593–2613, 2010 (GLOFs). The assessment of the regional and global impact of glacier changes in 5 the Himalaya is, however, hampered by a lack of mass balance data for most of the Himalayan glacier range. Multi-temporal digital terrain models (DTMs) allow glacier mass balance to be mass changes calculated since the availability of stereo imagery. -

Durham E-Theses
Durham E-Theses The ecology and feeding behaviour of the himalayan the (hemitragus jemlahicus) in the Langtang valley, Nepal Green, Michael J. B. How to cite: Green, Michael J. B. (1978) The ecology and feeding behaviour of the himalayan the (hemitragus jemlahicus) in the Langtang valley, Nepal, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/8982/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 THE ECOLOGY AND FEEDING BEHAVIOUR OF THE HIMALAYAN TAHR (HEMITRAGUS JEMLAHICUS) IN THE LANGTANG VALLEY, NEPAL by Michael J. B. Green B.Sc. Submitted to the University of Durham for the degree of Master of Science Aug\ist 1978 The cop5Tight of this thesis rests with the author. No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. -

Ecology and Conservation of Mountain Ungulates in Great Himalayan National Park, Western Himalaya
FREEP-GHNP 03/10 Ecology and Conservation of Mountain Ungulates in Great Himalayan National Park, Western Himalaya Vinod T. R. and S. Sathyakumar Wildlife Institute of India, Post Box No. 18, Chandrabani, Dehra Dun – 248 001, U.P., INDIA December 1999 ACKNOWLEDGEMENTS We are thankful to Shri. S.K. Mukherjee, Director, Wildlife Institute of India, for his support and encouragement and Shri. B.M.S. Rathore, Principal Investigator of FREEP-GHNP Project, for valuable advice and help. We acknowledge the World Bank, without whose financial support this study would have been difficult. We are grateful to Dr. Rawat, G.S. for his guidance and for making several visits to the study site. We take this opportunity to thank the former Principal Investigator and present Director of GHNP Shri. Sanjeeva Pandey. He is always been very supportive and came forward with help- ing hands whenever need arised. Our sincere thanks are due to all the Faculty members, especially Drs. A.J.T. Johnsingh, P.K. Mathur, V.B. Mathur, B.C. Choudhary, S.P. Goyal, Y.V. Jhala, D.V.S. Katti, Anil Bharadwaj, R. Chundawat, K. Sankar, Qamar Qureshi, for their sug- gestions, advice and help at various stages of this study. We are extremely thankful to Shri. S.K. Pandey, PCCF, HP, Shri. C.D. Katoch, former Chief Wildlife Warden, Himachal Pradesh and Shri. Nagesh Kumar, former Director GHNP, for grant- ing permission to work and for providing support and co-operation through out the study. We have been benefited much from discussions with Dr. A.J. Gaston, Dr. -

Himalayan Tahr in New Zealand Factsheet
Himalayan tahr in New Zealand July 2020 What is the Himalayan Thar Control Plan 1993? The management of Himalayan tahr is governed by a statutory plan, the Himalayan Thar Control Plan 1993, prepared under section 5(1)(d) of the Wild Animal Control Act 1977 (www.doc.govt.nz/himalayan-thar-control-plan). A key element of the Himalayan Thar Control Plan is that it sets a maximum population of 10,000 tahr across all land tenures in the tahr feral range (the legal boundary of where tahr are allowed to be). Tahr are officially controlled within seven tahr management units: The seven tahr management Bull (male) tahr. Photo: DOC units collectively comprise Himalayan tahr (Hemitragus jemlahicus) are large 706,000 ha goat-like animals, native to the central Himalayan of land inside the tahr ranges of India and Nepal. feral range (see map). Tahr were first released in New Zealand at Aoraki/Mt Tahr can be hunted on Cook in 1904 for recreational hunting and to attract overseas hunters. The males are known as bulls and 573,000 ha of public conservation land (PCL) prized as trophies by hunters. inside the tahr management units. With no natural predators in New Zealand, tahr quickly adapted to our alpine environment and have had a considerable impact on native vegetation. Significant numbers of tahr are now present in the central Southern Alps/Ka tiritiri o te Moana. (See map on back page). Photo: Dylan Higgison Female and juvenile tahr. Female and juvenile tahr. How many Himalayan tahr are there? There are very few tahr in the exclusion zones, as all tahr present in these areas are targeted for removal to prevent After three summer seasons of tahr population the tahr feral range from expanding. -

Status and Ecology of the Nilgiri Tahr in the Mukurthi National Park, South India
Status and Ecology of the Nilgiri Tahr in the Mukurthi National Park, South India by Stephen Sumithran Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Fisheries and Wildlife Sciences APPROVED James D. Fraser, Chairman Robert H. Giles, Jr. Patrick F. Scanlon Dean F. Stauffer Randolph H. Wynne Brian R. Murphy, Department Head July 1997 Blacksburg, Virginia Status and Ecology of the Nilgiri Tahr in the Mukurthi National Park, South India by Stephen Sumithran James D. Fraser, Chairman Fisheries and Wildlife Sciences (ABSTRACT) The Nilgiri tahr (Hemitragus hylocrius) is an endangered mountain ungulate endemic to the Western Ghats in South India. I studied the status and ecology of the Nilgiri tahr in the Mukurthi National Park, from January 1993 to December 1995. To determine the status of this tahr population, I conducted foot surveys, total counts, and a three-day census and estimated that this population contained about 150 tahr. Tahr were more numerous in the north sector than the south sector of the park. Age-specific mortality rates in this population were higher than in other tahr populations. I conducted deterministic computer simulations to determine the persistence of this population. I estimated that under current conditions, this population will persist for 22 years. When the adult mortality was reduced from 0.40 to 0.17, the modeled population persisted for more than 200 years. Tahr used grasslands that were close to cliffs (p <0.0001), far from roads (p <0.0001), far from shola forests (p <0.01), and far from commercial forestry plantations (p <0.001). -

ACE Appendix
CBP and Trade Automated Interface Requirements Appendix: PGA August 13, 2021 Pub # 0875-0419 Contents Table of Changes .................................................................................................................................................... 4 PG01 – Agency Program Codes ........................................................................................................................... 18 PG01 – Government Agency Processing Codes ................................................................................................... 22 PG01 – Electronic Image Submitted Codes .......................................................................................................... 26 PG01 – Globally Unique Product Identification Code Qualifiers ........................................................................ 26 PG01 – Correction Indicators* ............................................................................................................................. 26 PG02 – Product Code Qualifiers ........................................................................................................................... 28 PG04 – Units of Measure ...................................................................................................................................... 30 PG05 – Scientific Species Code ........................................................................................................................... 31 PG05 – FWS Wildlife Description Codes ...........................................................................................................