Status and Ecology of the Nilgiri Tahr in the Mukurthi National Park, South India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arabian Ungulate CAMP & Leopard, Tahr, and Oryx PHVA Final Report 2001.Pdf
Conservation Assessment and Management Plan (CAMP) For The Arabian Ungulates and Leopard & Population and Habitat Viability Assessment (PHVA) For the Arabian Leopard, Tahr, and Arabian Oryx 1 © Copyright 2001 by CBSG. A contribution of the IUCN/SSC Conservation Breeding Specialist Group. Conservation Breeding Specialist Group (SSC/IUCN). 2001. Conservation Assessment and Management Plan for the Arabian Leopard and Arabian Ungulates with Population and Habitat Viability Assessments for the Arabian Leopard, Arabian Oryx, and Tahr Reports. CBSG, Apple Valley, MN. USA. Additional copies of Conservation Assessment and Management Plan for the Arabian Leopard and Arabian Ungulates with Population and Habitat Viability Assessments for the Arabian Leopard, Arabian Oryx, and Tahr Reports can be ordered through the IUCN/SSC Conservation Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124. USA. 2 Donor 3 4 Conservation Assessment and Management Plan (CAMP) For The Arabian Ungulates and Leopard & Population and Habitat Viability Assessment (PHVA) For the Arabian Leopard, Tahr, and Arabian Oryx TABLE OF CONTENTS SECTION 1: Executive Summary 5. SECTION 2: Arabian Gazelles Reports 18. SECTION 3: Tahr and Ibex Reports 28. SECTION 4: Arabian Oryx Reports 41. SECTION 5: Arabian Leopard Reports 56. SECTION 6: New IUCN Red List Categories & Criteria; Taxon Data Sheet; and CBSG Workshop Process. 66. SECTION 7: List of Participants 116. 5 6 Conservation Assessment and Management Plan (CAMP) For The Arabian Ungulates and Leopard & Population and Habitat Viability Assessment (PHVA) For the Arabian Leopard, Tahr, and Arabian Oryx SECTION 1 Executive Summary 7 8 Executive Summary The ungulates of the Arabian peninsula region - Arabian Oryx, Arabian tahr, ibex, and the gazelles - generally are poorly known among local communities and the general public. -

Keshav Ravi by Keshav Ravi
by Keshav Ravi by Keshav Ravi Preface About the Author In the whole world, there are more than 30,000 species Keshav Ravi is a caring and compassionate third grader threatened with extinction today. One prominent way to who has been fascinated by nature throughout his raise awareness as to the plight of these animals is, of childhood. Keshav is a prolific reader and writer of course, education. nonfiction and is always eager to share what he has learned with others. I have always been interested in wildlife, from extinct dinosaurs to the lemurs of Madagascar. At my ninth Outside of his family, Keshav is thrilled to have birthday, one personal writing project I had going was on the support of invested animal advocates, such as endangered wildlife, and I had chosen to focus on India, Carole Hyde and Leonor Delgado, at the Palo Alto the country where I had spent a few summers, away from Humane Society. my home in California. Keshav also wishes to thank Ernest P. Walker’s Just as I began to explore the International Union for encyclopedia (Walker et al. 1975) Mammals of the World Conservation of Nature (IUCN) Red List species for for inspiration and the many Indian wildlife scientists India, I realized quickly that the severity of threat to a and photographers whose efforts have made this variety of species was immense. It was humbling to then work possible. realize that I would have to narrow my focus further down to a subset of species—and that brought me to this book on the Endangered Mammals of India. -

THE NILGIRIS Kms from Ooty and Kotagiri 31 Kms from Ooty, Are the Three Hill Stations of This District
THE NILGIRIS kms from Ooty and Kotagiri 31 kms from Ooty, are the three hill stations of this district. Geographical Location • The Nilgiris is situated at an elevation of 900 to 2636 meters above MSL. • The Nilgiris is bounded on North by Karnataka State on the East by Coimbatore District, Erode District, South by Coimbatore District and Kerala State and as the West by Kerala State. Important places District Collector: Tmt. J. Innocent Divya • Doddabetta - 2,623 mts above MSL - I.A.S highest Peak in the Tamil Nadu. • The Nilgiri Mountain Train-One among the three Mountain Railways of India designated as a UNESCO World Heritage Site. Three railways, the Darjeeling Himalayan Railway, the Nilgiri Mountain Railway, and the Kalka– Shimla Railway, are collectively designated as a UNESCO World Heritage Site under the name Mountain Railways of India. The fourth railway, the Matheran Hill Railway, is on the tentative list of UNESCO World Heritage Sites. REVENUE DIVISIONS: • Mudumalai National Park UDHAGAI • Pykara Waterfalls and the Ooty Lake COONOOR • Botanical Garden – Ooty GUDALUR • Rose Garden - Ooty HISTORY: • Ooty Lake and Boat House • The Name ‘Nilgiris’ means Blue hills the first mention of this name has been found • Raj Bhavan - Ooty in the Silappadikaram. • Dolphin's Nose - Coonoor • One of the oldest mountain ranges, located at the tri-junction of Tamil Nadu, Kerala • Lamb's rock - Coonoor and Karnataka. • Glenmorgan - Ooty • Nilgiris is a part of the Western Ghats. Ooty the “Queen of Hill Stations”, Coonoor 19 • Avalanche - Ooty For any queries mail to: [email protected] Forest • South Western ghats - Nilgiri tahrs are found only in the montane grasslands of the Southwestern Ghats. -
FORM 1 M 1. Basic Information
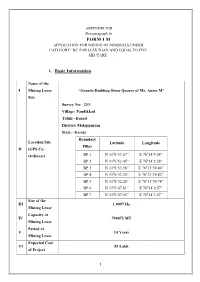
APPENDIX VIII (See paragraph 6) FORM 1 M APPLICATION FOR MINING OF MINERALS UNDER CATEGORY ‘B2’ FOR LESS THAN AND EQUAL TO FIVE HECTARE. 1. Basic Information Name of the I Mining Lease “Granite Building Stone Quarry of Mr. Anees M” Site Survey No:- 23/1 Village: Pandikkad Taluk:- Ernad District: Malappuram State:- Kerala Boundary Location/Site Latitude Longitude Pillar II (GPS Co- 0 0 Ordinate) BP 1 N 11 6’53.67” E 76 14’4.08” BP 2 N 1106’53.45” E 76014’2.38” BP 3 N 1106’53.58” E 76013’59.80” BP 4 N 1106’53.25” E 76013’59.82” BP 5 N 1106’52.26” E 76013’59.78” BP 6 N 1106’47.81” E 76014’0.57” BP 7 N 1106’47.56” E 76014’3.47” Size of the III 1.9997 Ha Mining Lease Capacity of IV 794075 MT Mining Lease Period of V 10 Years Mining Lease Expected Cost VI 85 Lakh of Project 1 Name Mr.Anees M Address S/o Muhammed Mampally House Contact VII Kodasseri Information Velluvangad P.O Malappuram Mob 9645126712 2. Environment Sensitivity Sl.No. Areas Distance (KM) Distance of project site from Rail, Road Kakathod Bridge-2.58km-NE 1 Bridge over the concerned River, Rivulet, Nellikuth-Vattathippara Bridge-4.79km-NW Nallah etc. Oravampuram Bridge-3.46km-SW Distance from infrastructural facilities Railway Line Thuvvur Railway Station -5.8 km -NE National Highway NH 966(Palakkad-Kozhikode)-290m W State Highway SH 73(Valachery- Nilambur)-300m-W Major District Road Kalankavu-Eriyad Road - 0.16km - S Other Road Panchayath Road-0.13km-SE 2 Electrical Transmission Line or Pole Karaya-400m-W Avalanche Lake-45.7km-NE Canal/Check Dam/Reservoirs/Lake/Pond Emerald Lake-48.4km-NE Anakode Pond-1.31km-NE In-take for drinking water/pump house Pandikkad-4KM -SW In-take for Irrigation canal pumps NIL Areas protected under international conventions, national or local legislation for 3 None within 1KM from project site their ecological, landscape, cultural or others related value. -

Bhadra Voluntary Relocation India
BHADRA VOLUNTARY RELOCATION INDIA INDIA FOREWORD During my tenure as Director Project Tiger in the Ministry of Environment and Forests, Govt. of India, I had the privilege of participating in voluntary relocation of villages from Bhadra Tiger Reserve. As nearly two decades have passed, whatever is written below is from my memory only. Mr Yatish Kumar was the Field Director of Bhadra Tiger Reserve and Mr Gopalakrishne Gowda was the Collector of Chikmagalur District of Karnataka during voluntary relocation in Bhadra Tiger Reserve. This Sanctuary was notified as a Tiger Reserve in the year 1998. After the notification as tiger reserve, it was necessary to relocate the existing villages as the entire population with their cattle were dependent on the Tiger Reserve. The area which I saw in the year 1998 was very rich in flora and fauna. Excellent bamboo forests were available but it had fire hazard too because of the presence of villagers and their cattle. Tiger population was estimated by Dr. Ullas Karanth and his love for this area was due to highly rich biodiversity. Ultimately, resulted in relocation of all the villages from within the reserve. Dr Karanth, a devoted biologist was a close friend of mine and during his visit to Delhi he proposed relocation of villages. As the Director of Project Tiger, I was looking at voluntary relocation of villages for tribals only from inside Tiger Reserve by de-notifying suitable areas of forests for relocation, but in this case the villagers were to be relocated by purchasing a revenue land which was very expensive. -

Bushmeat Hunting and Extinction Risk to the World’S Rsos.Royalsocietypublishing.Org Mammals
Downloaded from http://rsos.royalsocietypublishing.org/ on October 26, 2017 Bushmeat hunting and extinction risk to the world’s rsos.royalsocietypublishing.org mammals 1,2 4,5 Research William J. Ripple , Katharine Abernethy , Matthew G. Betts1,2, Guillaume Chapron6, Cite this article: Ripple WJ et al.2016 Bushmeat hunting and extinction risk to the Rodolfo Dirzo7, Mauro Galetti8,9, Taal Levi1,2,3, world’s mammals R. Soc. open sci. 3: 160498. 10,11 12 http://dx.doi.org/10.1098/rsos.160498 Peter A. Lindsey , David W. Macdonald , Brian Machovina13, Thomas M. Newsome1,14,15,16, Carlos A. Peres17, Arian D. Wallach18, Received: 10 July 2016 Accepted: 20 September 2016 Christopher Wolf1,2 and Hillary Young19 1GlobalTrophic Cascades Program, Department of Forest Ecosystems and Society, 2Forest Biodiversity Research Network, Department of Forest Ecosystems and Society, and 3Department of Fisheries and Wildlife, Oregon State University, Corvallis, Subject Category: OR 97331, USA Biology (whole organism) 4School of Natural Sciences, University of Stirling, Stirling FK9 4LA, UK 5Institut de Recherche en Ecologie Tropicale, CENAREST, BP 842 Libreville, Gabon Subject Areas: 6Grimsö Wildlife Research Station, Department of Ecology, Swedish University of ecology Agricultural Sciences, 73091 Riddarhyttan, Sweden 7Department of Biology, Stanford University, Stanford, CA 94305, USA Keywords: 8Universidade Estadual Paulista (UNESP), Instituto Biociências, Departamento de wild meat, bushmeat, hunting, mammals, Ecologia, 13506-900 Rio Claro, São Paulo, Brazil extinction 9Department of Bioscience, Ecoinformatics and Biodiversity, Aarhus University, 8000 Aarhus, Denmark 10Panthera, 8 West 40th Street, 18th Floor, New York, NY 10018, USA 11 Author for correspondence: Mammal Research Institute, Department of Zoology and Entomology, University of William J. -

Bi-Monthly Outreach Journal of National Tiger Conservation Authority Government of India
BI-MONTHLY OUTREACH JOURNAL OF NATIONAL TIGER CONSERVATION AUTHORITY GOVERNMENT OF INDIA Volume 3 Issue 2 Jan-Feb 2012 TIGER MORTALITY 2011 AS REPORTED BY STATES Natural & other cause Accident Seizure Inside tiger reserve Outside tiger Eliminated by dept Poaching No. of tiger deaths reserve UTTARAKHAND 14 1 1 1 — 17 8 9 KERALA 3 — — 1 — 4 2 2 ASSAM 3 — — 2 1 6 4 2 MADHYA PRADESH 5 — — — — 5 4 1 RAJASTHAN 1 — — — — 1 1 — ORISSA 1 — — — — 1 1 — TAMIL NADU 3 — — — — 3 1 2 WEST BENGAL 3 — — — — 3 2 1 KARNATAKA 3 — — 3 — 6 6 — MAHARASHTRA 2 — 1 2 1 6 1 5 UTTAR PRADESH — — 1 — — 1 1 — CHHATTISGARH — — — 2 — 2 — 2 BIHAR 1 — — — — 1 — 1 TOTAL 39 1 3 11 2 56 31 25 * One old tiger trophy was seized in Delhi Volume 3 Evaluation Protocol EDITOR Issue 2 Status of Dr Rajesh Gopal Jan-Feb Monitoring tigers in Phase-IV 2012 Western EDITORIAL in tiger Ghats COORDINATOR reserves & Landscape S P YADAV source areas Pg 4 Pg 15 CONTENT COORDINATOR Inder MS Kathuria Photo Tiger FEEDBACK Feature Soldiers Assessment Annexe No 5 Camera Protection Management Bikaner House traps at force gets Effectiveness Shahjahan Road New Delhi work in going in Evaluation Kalakad TR Bandipur, P8 [email protected] Pg 14 Nagarhole Cover photo Pg 18 Bharat Goel BI-MONTHLY OUTREACH JOURNAL OF NATIONAL TIGER CONSERVATION AUTHORITY GOVERNMENT OF INDIA n o t e f r o m t h e e d i t o r THE new year, with all its freshness, tigers and its prey in each tiger reserves which would commenced with a new set of initiatives complement the once in four year snapshot assess- from NTCA. -

Contextual Water Targets Pilot Study Noyyal-Bhavani River Basin, India
CONTEXTUAL WATER TARGETS PILOT STUDY NOYYAL-BHAVANI RIVER BASIN, INDIA May 2019 Ashoka Trust for Research in Ecology and the Environment (ATREE) 1 Bangalore, India This publication is based on the project report submitted to the Pacific Institute, USA as the result of the study on contextual water targets in the Noyyal-Bhavani river basin, India. Study duration: October 2018 to April 2019 Financial support: Pacific Institute, USA Additional financial support: World Wide Fund for Nature-India (WWF-India). Authors: Apoorva R., Rashmi Kulranjan, Choppakatla Lakshmi Pranuti, Vivek M., Veena Srinivasan Suggested Citation: R. Apoorva, Kulranjan, R., Pranuti, C. L., Vivek, M., and Srinivasan, V. 2019. Contextual Water Targets Pilot Study: Noyyal-Bhavani River Basin. Bengaluru. Ashoka Trust for Research in Ecology and the Environment (ATREE). Front-cover Photo Caption: Noyyal outflows from the Orathupalayam dam, which had become a reservoir of polluted water for years. Front-cover Photo Credit: Apoorva R. (2019) Back-cover Photo Caption: Untreated sewage in a drain flows towards the River Noyyal near Tiruppur city, Tamil Nadu Back-cover Photo Credit: Rashmi Kulranjan (2019) Acknowledgement: We are grateful to Mr. Ganesh Shinde from ATREE for his help and guidance related to land use classification and GIS maps in this project. We would like to thank all the participants of the project consultative meeting held in Coimbatore in March 2019 for sharing their ideas and contributing to the discussion. We are thankful to Ms. Upasana Sarraju for proofreading -

Captivating Coonoor
CAPTIVATING COONOOR Come to this small, yet enchanting, hill town of Tamil Nadu. Look beyond what is visible, and the spectacular unexpectedly unfurls. Coonoor is one of the most popular Hill Stations in Tamil Nadu and works its way into the tourists' hearts like magic. Known for its green slopes of tea plantations, where the leaves are laden with morning dew, Coonoor is the perfect weekend getaway for those seeking a retreat from the hustle and bustle of the draining city life. This enchanting destination is situated 1,850 m above sea level, amongst the hills of the Nilgiris, and is home to some of the most beautiful places in the Southern part of India. It is also well-connected to the major cities, making it conveniently accessible. It is, however the natural abundance which makes it an ideal getaway for anyone. With its clear skies adorned with twinkling stars, and a pleasant climate with temperatures ranging from 15 to 25 oC, the serene and peaceful atmosphere disturbed by but the chirping of birds makes it the perfect place to relax, unwind, and escape. Nestled amongst the lush giris of Nilgiris, it is a world full of tranquillity and bliss. Read on to find out more about the bounty of experiences Coonoor has to offer… ACRES WILD CHEESE FARM: Acres Wild is a 22-acre, family-run organic cheesemaking farm in Coonoor near The Tiger Rock Tea Estate situated at an altitude of 6,000 ft. The goal of Acres Wild Cheese Farm is to shape an eco-friendly, holistic and self-sustaining lifestyle to grow their own food organically and share this experience with visitors at their cheese factory. -

Western Ghats & Sri Lanka Biodiversity Hotspot
Ecosystem Profile WESTERN GHATS & SRI LANKA BIODIVERSITY HOTSPOT WESTERN GHATS REGION FINAL VERSION MAY 2007 Prepared by: Kamal S. Bawa, Arundhati Das and Jagdish Krishnaswamy (Ashoka Trust for Research in Ecology & the Environment - ATREE) K. Ullas Karanth, N. Samba Kumar and Madhu Rao (Wildlife Conservation Society) in collaboration with: Praveen Bhargav, Wildlife First K.N. Ganeshaiah, University of Agricultural Sciences Srinivas V., Foundation for Ecological Research, Advocacy and Learning incorporating contributions from: Narayani Barve, ATREE Sham Davande, ATREE Balanchandra Hegde, Sahyadri Wildlife and Forest Conservation Trust N.M. Ishwar, Wildlife Institute of India Zafar-ul Islam, Indian Bird Conservation Network Niren Jain, Kudremukh Wildlife Foundation Jayant Kulkarni, Envirosearch S. Lele, Centre for Interdisciplinary Studies in Environment & Development M.D. Madhusudan, Nature Conservation Foundation Nandita Mahadev, University of Agricultural Sciences Kiran M.C., ATREE Prachi Mehta, Envirosearch Divya Mudappa, Nature Conservation Foundation Seema Purshothaman, ATREE Roopali Raghavan, ATREE T. R. Shankar Raman, Nature Conservation Foundation Sharmishta Sarkar, ATREE Mohammed Irfan Ullah, ATREE and with the technical support of: Conservation International-Center for Applied Biodiversity Science Assisted by the following experts and contributors: Rauf Ali Gladwin Joseph Uma Shaanker Rene Borges R. Kannan B. Siddharthan Jake Brunner Ajith Kumar C.S. Silori ii Milind Bunyan M.S.R. Murthy Mewa Singh Ravi Chellam Venkat Narayana H. Sudarshan B.A. Daniel T.S. Nayar R. Sukumar Ranjit Daniels Rohan Pethiyagoda R. Vasudeva Soubadra Devy Narendra Prasad K. Vasudevan P. Dharma Rajan M.K. Prasad Muthu Velautham P.S. Easa Asad Rahmani Arun Venkatraman Madhav Gadgil S.N. Rai Siddharth Yadav T. Ganesh Pratim Roy Santosh George P.S. -

Arabian Tahr in Oman Paul Munton
Arabian Tahr in Oman Paul Munton Arabian tahr are confined to Oman, with a population of under 2000. Unlike other tahr species, which depend on grass, Arabian tahr require also fruits, seeds and young shoots. The areas where these can be found in this arid country are on certain north-facing mountain slopes with a higher rainfall, and it is there that reserves to protect this tahr must be made. The author spent two years in Oman studying the tahr. The Arabian tahr Hemitragus jayakari today survives only in the mountains of northern Oman. A goat-like animal, it is one of only three surviving species of a once widespread genus; the other two are the Himalayan and Nilgiri tahrs, H. jemlahicus and H. hylocrius. In recent years the government of the Sultanate of Oman has shown great interest in the country's wildlife, and much conservation work has been done. From April 1976 to April 1978 I was engaged jointly by the Government, WWF and IUCN on a field study of the tahr's ecology, and in January 1979 made recommendations for its conservation, which were presented to the Government. Arabian tahr differ from the other tahrs in that they feed selectively on fruits, seeds and young shoots as well as grass. Their optimum habitat is found on the north-facing slopes of the higher mountain ranges of northern Oman, where they use all altitudes between sea level and 2000 metres. But they prefer the zone between 1000 and 1800m where the vegetation is especially diverse, due to the special climate of these north-facing slopes, with their higher rainfall, cooler temperatures, and greater shelter from the sun than in the drought conditions that are otherwise typical of this arid zone. -

Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26.