Dangerous Drugs Act
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genl:VE 1970 © World Health Organization 1970
Nathan B. Eddy, Hans Friebel, Klaus-Jiirgen Hahn & Hans Halbach WORLD HEALTH ORGANIZATION ORGANISATION .MONDIALE DE LA SANT~ GENl:VE 1970 © World Health Organization 1970 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. Nevertheless governmental agencies or learned and professional societies may reproduce data or excerpts or illustrations from them without requesting an authorization from the World Health Organization. For rights of reproduction or translation of WHO publications in toto, application should be made to the Division of Editorial and Reference Services, World Health Organization, Geneva, Switzerland. The World Health Organization welcomes such applications. Authors alone are responsible for views expressed in signed articles. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Director-General of the World Health Organization concerning the legal status of any country or territory or of its authorities, or concerning the delimitation of its frontiers. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. © Organisation mondiale de la Sante 1970 Les publications de l'Organisation mondiale de la Sante beneficient de la protection prevue par les dispositions du Protocole n° 2 de la Convention universelle pour la Protection du Droit d'Auteur. Les institutions gouvernementales et les societes savantes ou professionnelles peuvent, toutefois, reproduire des donnees, des extraits ou des illustrations provenant de ces publications, sans en demander l'autorisation a l'Organisation mondiale de la Sante. Pour toute reproduction ou traduction integrate, une autorisation doit etre demandee a la Division des Services d'Edition et de Documentation, Organisation mondiale de la Sante, Geneve, Suisse. -

Emergency Department Requirement for Assessment of Outpatients Who Receive Injectable Narcotics
This is an Interior Health CONTROLLED document. A copy of this document in paper form is not controlled and should be checked against the electronic file version to ensure accuracy Administrative Policy Manual Code: AH Patient/Client Relations/Care AH0600 - EMERGENCY DEPARTMENT REQUIREMENT FOR ASSESSMENT OF OUTPATIENTS WHO RECEIVE INJECTABLE NARCOTICS 1.0 PURPOSE To promote quality care and patient safety by ensuring Outpatients are appropriately assessed by an EDP or MRP when receiving or prior to receiving an injectable Narcotic in Emergency Departments (ED) of Interior Health (IH). 2.0 DEFINITIONS EDP Emergency Department Physician MRP Most Responsible Physician Narcotic Any substance set out in the schedule or anything that contains any substance set out in the schedule in the (Narcotic Control Regulations, CRC. C., 1041), see Appendix B. Outpatient A patient who presents to the ED for care and is not hospitalized or admitted to hospital. 3.0 POLICY 3.1 Assessment and Order Required This policy applies to Narcotic injection(s) administered to Outpatients being treated for acute and/or chronic conditions at IH EDs. Narcotic injection(s) will only be administered to Outpatients when the Outpatient has been assessed by an EDP/MRP (with active privileges in the administering facility) and the EDP/MRP has written an order dated within 24 hours of the Narcotic injection being administered. NOTE: Phone orders are acceptable on the undertaking the EDP/MRP will assess the Outpatient within 24 hours of the phone order. Orders for a series of Narcotic injections in excess of 24 hours require Outpatients be reassessed by an EDP/MRP (with active privileges in the administering facility) within 24 hours of the initial order and within every 24 hours thereafter while the Narcotic injection order is in effect. -

Guidelines for Ensuring Patient Access To, and Safe Management Of, Controlled Medicines Notice
African Palliative Care Association Guidelines for Ensuring Patient Access to, and Safe Management of, Controlled Medicines Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. APCA and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of this publication. However, in view of the possibility of human error or changes in sciences, neither APCA nor the publisher nor any other party who have been involved in the preparation or publication of this work warrants that the information contained in this publication is complete and correct and they disclaim all responsibility for any errors or omission or for the results obtained from use of the information contained herein. African Palliative Care Association Foreword The 59th Session of the World Health conventions. Several countries make the Assembly of the UN adopted resolution importation, storage, distribution and 58.22, thereby recognising the dispensing of controlled medicines more importance of improving pain relief restrictive than is needed. Additionally, using opioid analgesics and calling on it is a requirement for countries to member states to remove barriers to provide detailed annual estimates and their medical use and availability. reports for narcotic substances to the International Narcotic Control Board to While advances have been made pursuing procure or produce controlled medicines. this agenda in Africa (e.g. legalisation of However, formulating reliable estimates oral morphine prescription rights for nurses is often a barrier to accessing controlled and clinical officers in Uganda, approval medicines, while the procurement of access to morphine for hospices of opioids is subject to complex and in Zambia, and advocacy progress in lengthy exportation and importation Malawi and Kenya), challenges remain. -

Narcotic Drugs and Psychotropic Substances (Control) Act
LAWS OF GUYANA Narcotic Drugs and Psychotropic Cap. 35:11 3 Substances (Control) CHAPTER 35:11 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONTROL) ACT ARRANGEMENT OF SECTIONS PART I PRELIMINARY SECTION 1. Short title. 2. Interpretation. PART II PROHIBITION OF POSSESSION OF AND TRAFFICKING IN NARCOTICS AND CULTIVATION OF CERTAIN PLANTS 3. Definitions for Part II. 4. Penalty for possession of narcotic. 5. Penalty for trafficking in narcotic. 6. Penalty for supply, etc., of narcotic to child or young person if death results from consumption or administration of it. 7. Penalty for bringing into prison or taking out of prison, etc., of a narcotic. 8. Penalty for cultivation of certain plants. 9. Procedure for purposes of section 8(2) to (6). 10. Power of entry in respect of State or Government lands. 11. Power of destruction of prohibited plants. 12. Penalty for certain other acts connected with narcotics. 13. Certain prescriptions to be unlawful. 14. Penalty for receiving additional narcotic or prescription without disclosure of earlier receipt. 15. Removal of name from register. L.R.O. 3/1998 LAWS OF GUYANA 4 Cap. 35:11 Narcotic Drugs and Psychotropic Substances (Control) PART III NARCOTICS IN TRANSIT SECTION 16. Definitions for Part III. 17. Prohibition against sending narcotics by post. 18. Narcotics in transit. 19. Control of Comptroller over narcotics brought into Guyana in transit. 20. Tampering with narcotics in transit. 21. Diversion in Guyana of narcotics in transit. 22. Variations in export authorisation, import authorisation or diversion certificate granted in country other than Guyana. PART IV LICENCES 23. Grant and renewal of licences. -
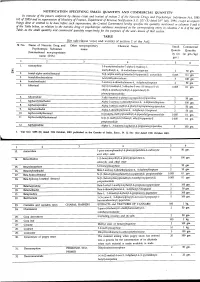
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

Questions & Answers
SERVING CANADIANS Research and Statistics Division questions & answers February 2003 www.canada.justice.gc.ca/en/ps/rs Drug Use and Offending by: Nathalie Quann, Research Analyst Q1. How has drug legislation changed recently? Table of Contents Q1. How has drug legislation changed recently? .................................................1 Since May 1997, the Controlled Drugs and Substances Act governs all drug offences in Canada. Q2. What are the most recent Canadian statistics on drug use? ............................2 Prior to 1997, the two most important federal statutes dealing with Q3. What is the public attitude towards illicit drugs were the Narcotics Control Act (NCA) and the Food and decriminalization of drugs? ...................4 Drugs Act (FDA). The Narcotics Control Act governed over 120 different types of drugs such as cocaine, heroin, opium, and Q4. What do Canadians think about the cannabis. The NCA did not distinguish one drug from another. For medical use of soft drugs? ......................5 example, cannabis and cocaine offenders were subject to the same Q5. How is the health care system affected criminal procedures and penalties. The Food and Drugs Act governed by drug use and abuse? ..........................5 the regulation of pharmaceutical drugs, food, cosmetics, and medical devices. Two parts most specifically dealt with the non-medical use Q6. How many drug offences were reported of specific drugs: Part III governed "controlled drugs" (such as by the police in 2000? ............................6 amphetamines, barbiturates, testosterone) while Part IV governed Q7. Are offenders always charged by the "restricted drugs" (such as LSD, and other hallucinogenic drugs). police when drugs are involved? ...........7 The maximum penalties were less strict under the FDA than the NCA. -

Supplement 1: Additional Tables and Figures
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplement 1: Additional tables and figures Box S1: Substances included and excluded from the International Narcotic Control Board (INCB) data on narcotic consumption, in alphabetical order. Opioids included in the opioid consumption calculation: 1. (+)-cis-3-methylfental 35. Bezitramide 2. 3-Acetylmorphine 36. Butyrfentanyl 3. 3-Methylfentanyl 37. Carfentanil 4. 3-Methylthiofentanyl 38. Carfentanyl 5. 3-Monoacetylmorphine 39. Clonitazene 6. 4-Fluoroisobutyrfentanyl 40. Codeine 7. 6-Acetylmorphine 41. Codeine-6GLUC 8. 6-Monoacetylmorphine 42. Codeine-6-glucuronide 9. Acetorphine 43. Codeine-Methyl 10. Acetyl-alpha-methylfentanyl 44. Codeine-N-oxide 11. Acetyldihydrocodeine 45. Codoxime 12. Acetylfentanyl 46. Conc. of poppy straw (C) ACA 13. Acetylmethadol 47. Conc. of poppy straw (C) AMA 14. Acetylmorphine 48. Conc. of poppy straw (C) AOA 15. Acrylfentanyl 49. Conc. of poppy straw (C) ATA 16. AH-7921 50. Conc. of poppy straw (C) GW 17. Alfentanil 51. Conc. of poppy straw (M) ACA 18. Allylprodine 52. Conc. of poppy straw (M) AMA 19. Alphacetylmethadol 53. Conc. of poppy straw (M) AOA 20. Alphameprodine 54. Conc. of poppy straw (M) ATA 21. Alphamethadol 55. Conc. of poppy straw (M) GW 22. alpha-Methylfentanyl 56. Conc. of poppy straw (N) GW 23. alpha-Methylthiofentanyl 57. Conc. of poppy straw (O) 24. Alphaprodine 58. Conc. of poppy straw (O) ACA 25. Anileridine 59. Conc. of poppy straw (O) AMA 26. Benzethidine 60. Conc. of poppy straw (O) AOA 27. -

Controlled Drugs and Substances Act Loi Réglementant Certaines
CANADA CONSOLIDATION CODIFICATION Controlled Drugs and Loi réglementant Substances Act certaines drogues et autres substances S.C. 1996, c. 19 L.C. 1996, ch. 19 Current to October 27, 2015 À jour au 27 octobre 2015 Last amended on July 16, 2015 Dernière modification le 16 juillet 2015 Published by the Minister of Justice at the following address: Publié par le ministre de la Justice à l’adresse suivante : http://laws-lois.justice.gc.ca http://lois-laws.justice.gc.ca OFFICIAL STATUS CARACTÈRE OFFICIEL OF CONSOLIDATIONS DES CODIFICATIONS Subsections 31(1) and (2) of the Legislation Les paragraphes 31(1) et (2) de la Loi sur la Revision and Consolidation Act, in force on révision et la codification des textes législatifs, June 1, 2009, provide as follows: en vigueur le 1er juin 2009, prévoient ce qui suit : Published 31. (1) Every copy of a consolidated statute or 31. (1) Tout exemplaire d'une loi codifiée ou d'un Codifications consolidation is consolidated regulation published by the Minister règlement codifié, publié par le ministre en vertu de comme élément evidence under this Act in either print or electronic form is ev- la présente loi sur support papier ou sur support élec- de preuve idence of that statute or regulation and of its contents tronique, fait foi de cette loi ou de ce règlement et de and every copy purporting to be published by the son contenu. Tout exemplaire donné comme publié Minister is deemed to be so published, unless the par le ministre est réputé avoir été ainsi publié, sauf contrary is shown. -

0 Cover Part Two\374
PART TWO DEUXIÈME PARTIE SEGUNDA PARTE ﺍﳉﺰﺀ ﺍﻟﺜﺎﱐ 第二部分 ЧАСТЬ ВТΟΡАЯ - (...), [...] - (-)-3-hidroxi-N- (-)-(2R)-N-méthyl-1- fenacilmorfinán → Levophenacyl-morphan phénylpropan-2-amine → Levometamfetamine (-)-3-hidroxi-N- (-)-(3S,6R)-6- metilmorfinán → Levorphanol (dimethylamino)-4,4- (-)-3-hydroksymorfinan → Norlevorphanol diphenyl-3-heptanol → Betamethadol (-)-3-hydroksy-N- (-)-(3S,6R)-6- fenacylmorfinan → Levophenacyl-morphan (dimethylamino)-4,4- (-)-3-hydroksy-N- diphenyl-3-heptanol metylmorfinan → Levorphanol acetate (ester) → Betacetylmethadol (-)-3-hydroxymorphinan → Norlevorphanol (-)-(5R)-4,5-epoxy-3- (-)-3-hydroxy-N- methoxy-9α-methyl methylmorphinan → Levorphanol → Thebacon morphin-6-en-6-yl acetate (-)-3-hydroxynormorphinan → Norlevorphanol (-)-(5R,6S)-3-benzyloxy-4,5- (-)-3-hydroxy-N- epoxy-9a-methylmorphin- phenacylmorphinan → Levophenacyl-morphan 7-en-6-yl myristate → Myrophine (-)-3-hydroxytropane-2- (-)-(5R,6S)-4,5-epoxy carboxylat → Ecgonine morphin-7-en-3,6-diol → Normorphine (-)-3-idrossi-N- (-)-(5R,6S,14S)-4,5-epoxy- metilmorfinano → Levorphanol 14-hydroxy-3-methoxy- (-)-3-methoxy-N- 9a-methylmorphinan-6- methylmorphinan → Levomethorphan one → Oxycodone (-)-3-metil-2,2-difenil-4- (-)-(R)-4,5-epoxy-3- morfolinbutirilpirrolidina → Levomoramide methoxy-9a-methyl (-)-3-metoksy-N- morphinan-6-one → Hydrocodone metylmorfinan → Levomethorphan (-)-(R)-6-(dimethylamino)- (-)-3-metossi-N-metil- 4,4-diphenyl-3-heptanone → l-methadone morfinano → Levomethorphan (-)-(R)-N,α-dimethyl (-)-3-metoxi-N- phenethylamine → Levometamfetamine -

ATP, 489 Absolute Configuration Benzomotphans, 204 Levotphanol
Index AIDA, 495 Affinity labeling, analogs of (Cont.) cAMP, 409, 489 motphine,448 ATP, 409, 489 naltrexone, 449 [3H] ATP, 489 norlevotphanol,449 Absolute configuration normetazocine, 181 benzomotphans, 204 norpethidine, 232 levotphanol, 115 oripavine, 453 methadone and analogs, 316 oxymotphone, 449 motphine, 86 K-Agonists, 179,405,434 phenoperidine, 234 Aid in Interactive Drug Analysis, 495 piperazine derivatives, 399 [L-Ala2] dermotphin, 363 prodines and analogs, 272 [D-Ala, D-Leu] enkephalin (DADL), 68, 344 sinomenine, 28, 115 [D-Ala2 , Bugs] enkephalinamide, 347, 447 viminol, 400 [D-Ala2, Met'] enkephalinamide, 337, 346, Ac 61-91,360 371,489 Acetylcholine, 5, 407 [D-Ala2]leu-enkephalin, 344, 346, 348 Acetylcholine analogs, 186, 191 [D-Ala2] met-enkephalin, 348 l-Acetylcodeine, 32 [D-Ala2] enkephalins, 347 Acetylmethadols (a and (3) Alfentanil, 296 maintenance of addicts by a-isomer, 304, 309 (±)-I1(3-Alkylbenzomotphans, 167, 170 metabolism, 309 11(3-Alkylbenzomotphans, 204 N-allyl and N-CPM analogs, 310, 431 7-Alkylisomotphinans, 146 stereochemistry, 323 N-Alkylnorketobemidones, 431 synthesis, 309 N-Alkylnorpethidines, 233 X-ray crystallography, 327 N-Allylnormetazocine, 420 6-Acetylmotphine, receptor binding, 27 N-Allylnormotphine, 405 Acetylnormethadol, 323 N-Allylnorpethidine, 233 8(3-Acyldihydrocodeinones, 52 3-Allylprodines (a and (3), 256 14-Acyl-4,5-epoxymotphinans, 58 'H-NMR and stereochemistry, 256 7-Acylhydromotphones, 128 X-ray crystallography, 256 Addiction, 4 N-Allylnormetazocine, 420 Adenylate cyclase, 6, 409, 413, 424, -

Laws and Regulations Promulgated to Give Effect to the Provisions of the International Treaties on Narcotic Drugs and Psychotropic Substances
E/NL. 1976/3 12 December 1978 Mf UNITED NATIONS ENGLISH AND SPANISH ONLY Original: SPANISH LAWS AND REGULATIONS PROMULGATED TO GIVE EFFECT TO THE PROVISIONS OF THE INTERNATIONAL TREATIES ON NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES CHILE Communicated by the Government of Chile NOTE BY THE SECRETARY GENERAL - In accordance with the relevant Articles of the International Treaties on Narcotic Drugs and Psychotropic Substances, the Secretary-General has the honour to communicate the following legislative text. Ministry of Public Health Directorate General of Health Technical Department ADDS THE DRUGS SPECIFIED TO THOSE ENUMERATED IN ARTICLE 2 OP THE NARCOTIC DRUGS REGULATIONS, SUPREME DECREE No. 137, of I96O 1/ No. 10,676 Santiago, 26 December 1974* Considering: that the Narcotic Drugs Regulations, Supreme Decree No. 137? of 12 March I96O l/ mention, in article 2 the substances which are deemed to be narcotic drugs and in article 4 authorize the Director General of Health to add to those enumerated in the said article 2, by a resolution to be published in the "Diario Oficial", any new drug or preparation of similar therapeutic action; that the list given in the said article 2 of the aforementioned Regulations and in the supplementary orders of later date does not include all the drugs which are now classified as narcotic drugs; that the latter should be considered to include those substances placed under international control by virtue of the corresponding Conventions and, in particular, the Single Convention on Narcotic Drugs, 1961, approved by the National Congress of Chile and adopted for enforcement as a law of the Republic by Supreme Decree No. -

New Drug Substances with Abuse Potential
New drug substances with abuse potential: Points to consider for the development and marketing - Regulatory environment in the European Union and the United States - Wissenschaftliche Prüfungsarbeit zur Erlangung des Titels „Master of Drug Regulatory Affairs“ der Mathematisch-Naturwissenschaftlichen Falkultät der Rheinischen Friedrich-Wilhems-Universität Bonn vorgelegt von Dr. Silke Jung Bonn 2006 Betreuer und 1. Referent: Dr. Winfried Kleinert, Bundesopiumstelle (BfArM) Zweiter Referent: RA Burkhard Stäter, Anwaltskanzlei Sträter To my husband - for his patience and support - Danksagung An erster Stelle möchte ich mich ganz herzlich bei Herrn Dr. Winfried Kleinert und Herrn RA Burkhard Sträter für die Betreuung und Unterstützung meiner Arbeit bedanken. Mein besonderer Dank gilt auch Herrn Dr. Joachim Heinze und Herrn Dr. Burkahrd Daldrup, die mir den Besuch des ergänzenden Studiengangs „Master of Drug Regulatory Affairs“ ermöglicht haben. Für die kritische Durchsicht meiner Arbeit und die vielen hilfreichen Anmerkungen danke ich meine Kolleginnen Dr. Gesine Bejeuhr, Dr. Kim Goldenstein, Dr. Maren Großmüller, Dr. Carol Huntington und Dr. Barbara Römer. Besonderer Dank gilt nicht zuletzt auch meiner Familie, vor allem meinem Mann Dr. Guido Thömmes, die meine Arbeit mit Liebe und Unterstützung begleitet hat. TABLE OF CONTENTS A1 Table of contents Table of contents......................................................................................................................... A1 Abbreviations.............................................................................................................................