List of Narcotic Drugs Under International Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Minnesota Statutes 1979 Supplement
MINNESOTA STATUTES 1979 SUPPLEMENT 152.01 PROHIBITED DRUGS CHAPTER 152. PROHIBITED DRUGS Sec. 152.01 Definitions. 152.02 Schedules of controlled substances; admin istration of chapter. 152.01 Definitions. [For text of subds 1 to 8, see M.S.1978] Subd. 9. Marijuana. "Marijuana" means all parts of the plant of any species of the genus Cannabis, including all agronomical varieties, whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds or resin, but shall not include the mature stalks of such plant, fiber from such stalks, oil or cake made from the seeds of such plant, any other compound, manufacture, salt, derivative, mix ture, or preparation of such mature stalks, except the resin extracted therefrom, fiber, oil, or cake, or the sterilized seed of such plant which is incapable of germination. [For text of subds 10 to 17, see M.S.1978] [ 1979 c 157 s 1 ] 152.02 Schedules of controlled substances; administration of chapter. [For text of subd 1, see M.S.1978) Subd. 2. The following items are listed in Schedule I: (1) Any of the following substances, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, whenever the exis tence of such isomers, esters, ethers and salts is possible within the specific chemical des ignation: Acetylmethadol; Allylprodine; Alphacetylmethadol; Alphameprodine; Alpham- ethadol; Benzethidine; Betacetylmethadol; Betameprodine; Betamethadol; Betaprodine; Clonitazene; Dextromoramide; Dextrorphan; Diampromide; Diethyliambutene; Dime- noxadol; Dimepheptanol; Dimethyliambutene; Dioxaphetyl butyrate; Dipipanone; Ethylmethylthiambutene; Etonitazene; Etoxeridine; Furethidine; Hydroxypethidine; Ke- tobemidone; Levomoramide; Levophenacylmorphan; Morpheridine; Noracymethadol; Norlevorphanol; Normethadone; Norpipanone; Phenadoxone; Phenampromide; Pheno- morphan; Phenoperidine; Piritramide; Proheptazine; Properidine; Racemoramide; Tri meperidine. -

CONTROLLED SUBSTANCE, DRUG, DEVICE and COSMETIC ACT - SCHEDULE I CONTROLLED SUBSTANCES Act of Jun
CONTROLLED SUBSTANCE, DRUG, DEVICE AND COSMETIC ACT - SCHEDULE I CONTROLLED SUBSTANCES Act of Jun. 23, 2011, P.L. 36, No. 7 Cl. 35 Session of 2011 No. 2011-7 SB 1006 AN ACT Amending the act of April 14, 1972 (P.L.233, No.64), entitled "An act relating to the manufacture, sale and possession of controlled substances, other drugs, devices and cosmetics; conferring powers on the courts and the secretary and Department of Health, and a newly created Pennsylvania Drug, Device and Cosmetic Board; establishing schedules of controlled substances; providing penalties; requiring registration of persons engaged in the drug trade and for the revocation or suspension of certain licenses and registrations; and repealing an act," further providing for Schedule I controlled substances. The General Assembly of the Commonwealth of Pennsylvania hereby enacts as follows: Section 1. Section 4(1) of the act of April 14, 1972 (P.L.233, No.64), known as The Controlled Substance, Drug, Device and Cosmetic Act, amended November 24, 1999 (P.L.894, No.55), is amended to read: Section 4. Schedules of Controlled Substances.--The following schedules include the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. (1) Schedule I--In determining that a substance comes within this schedule, the secretary shall find: a high potential for abuse, no currently accepted medical use in the United States, and a lack of accepted safety for use under medical supervision. The following controlled substances are included in this schedule: (i) Any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, whenever the existence of such isomers, esters, ethers and salts is possible within the specific chemical designation: 1. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Thieme: Pharmakologie Und Toxikologie
627 Sachverzeichnis Vorbemerkung: Im Text des Buches sind für Wirkstoffe immer die internationalen Freinamen benutzt. Im Register sind die Handels- namen mit gekennzeichnet. Die Handelsnamen sind in den Tabellen „Notwendige Wirkstoffe“ aufgelistet. A Acetyldigoxin 149 Adenosin 139, 163 – Struktur 145 – als Antiarrhythmikum 162 Abacavir 532, 534 Acetylierer, langsamer 59 – Schmerzempfindung 289 Abarelix 399f Acetylsalicylsäure 310, 314ff – Struktur 139 Abatacept 331 – bei akutem rheumatischen Fieber 324 Adenosindesaminase 462 – bei rheumatoider Arthritis 322 – bei Angina pectoris 182 Adenosindiphosphat 139 ABC-Transporter 30 – Anwendung 314 Adenosinmonophosphat, zyklisches 99f Abciximab 183, 209 – bei Herzinfarkt 184 Adenosin-Nukleotid 139 – Thrombozytenaggregationshemmung – Kontraindikation 316 Adenosin-Rezeptor 139 207 – Metabolismus 315 Adenosintriphosphat 139 Abführmittel-Colon 246 – bei Migräne 307 – Schmerzempfindung 289 Abhängigkeit 49 – Nebenwirkung 315 – als Überträgersubstanz 139 – Hypnotika 363 – Pharmakokinetik 314f Adenylatcyclase 8, 100 Abilify 346 – Re-Infarkt-Prophylaxe 184 Adermin 271 Absence – bei Schlaganfall 186 Adevofir-dipivoxil bei Hepatitis B 253 – Antiepileptikawahl 375 – Schmerztherapie, Stufenschema 305 ADH (antidiuretisches Hormon) s. Adiu- – pyknoleptische, Antiepileptikawahl – Schwangerschaft 316 retin 375 – Struktur 315 Adhäsionsprotein 333 Absorption s. Resorption – Thromboseprophylaxe 209 ADHS (Aufmerksamkeits-Defizit/Hyper- Abstillen 404 – Thrombosetherapie 209 aktivitäts-Syndrom) 362 Acamprosat bei -

Drugs of Abuseon September Archived 13-10048 No
U.S. DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION WWW.DEA.GOV 9, 2014 on September archived 13-10048 No. v. Stewart, in U.S. cited Drugs of2011 Abuse EDITION A DEA RESOURCE GUIDE V. Narcotics WHAT ARE NARCOTICS? Also known as “opioids,” the term "narcotic" comes from the Greek word for “stupor” and originally referred to a variety of substances that dulled the senses and relieved pain. Though some people still refer to all drugs as “narcot- ics,” today “narcotic” refers to opium, opium derivatives, and their semi-synthetic substitutes. A more current term for these drugs, with less uncertainty regarding its meaning, is “opioid.” Examples include the illicit drug heroin and pharmaceutical drugs like OxyContin®, Vicodin®, codeine, morphine, methadone and fentanyl. WHAT IS THEIR ORIGIN? The poppy papaver somniferum is the source for all natural opioids, whereas synthetic opioids are made entirely in a lab and include meperidine, fentanyl, and methadone. Semi-synthetic opioids are synthesized from naturally occurring opium products, such as morphine and codeine, and include heroin, oxycodone, hydrocodone, and hydromorphone. Teens can obtain narcotics from friends, family members, medicine cabinets, pharmacies, nursing 2014 homes, hospitals, hospices, doctors, and the Internet. 9, on September archived 13-10048 No. v. Stewart, in U.S. cited What are common street names? Street names for various narcotics/opioids include: ➔ Hillbilly Heroin, Lean or Purple Drank, OC, Ox, Oxy, Oxycotton, Sippin Syrup What are their forms? Narcotics/opioids come in various forms including: ➔ T ablets, capsules, skin patches, powder, chunks in varying colors (from white to shades of brown and black), liquid form for oral use and injection, syrups, suppositories, lollipops How are they abused? ➔ Narcotics/opioids can be swallowed, smoked, sniffed, or injected. -

Federal Register/Vol. 84, No. 26/Thursday, February 7, 2019/Notices
2570 Federal Register / Vol. 84, No. 26 / Thursday, February 7, 2019 / Notices National Fire Protection Association Register pursuant to Section 6(b) of the Morrissette Drive, Springfield, Virginia (‘‘NFPA’’) has filed written notifications Act on October 22, 2018 (83 FR 53297). 22152. simultaneously with the Attorney Suzanne Morris, SUPPLEMENTARY INFORMATION: General and the Federal Trade The Commission disclosing additions or Chief, Premerger and Division Statistics Unit, Attorney General has delegated his changes to its standards development Antitrust Division. authority under the Controlled activities. The notifications were filed [FR Doc. 2019–01489 Filed 2–6–19; 8:45 am] Substances Act to the Administrator of for the purpose of extending the Act’s BILLING CODE 4410–11–P the Drug Enforcement Administration provisions limiting the recovery of (DEA), 28 CFR 0.100(b). Authority to antitrust plaintiffs to actual damages exercise all necessary functions with under specified circumstances. DEPARTMENT OF JUSTICE respect to the promulgation and implementation of 21 CFR part 1301, Specifically, NFPA has provided an Drug Enforcement Administration updated and current list of its standards incident to the registration of development activities, related technical [Docket No. DEA–392] manufacturers, distributors, dispensers, committee and conformity assessment importers, and exporters of controlled activities. Information concerning NFPA Bulk Manufacturer of Controlled substances (other than final orders in regulations, technical committees, -

SB566 1 119787-1 2 by Senators Orr, Erwin, Sanford, and Butler 3 RFD
1 SB566 2 119787-1 3 By Senators Orr, Erwin, Sanford, and Butler 4 RFD: Judiciary 5 First Read: 23-MAR-10 Page 0 1 119787-1:n:03/08/2010:DA/mfp LRS2010-1714 2 3 4 5 6 7 8 SYNOPSIS: Under existing law, salvia divinorum and 9 Salvinorin A are not listed as controlled 10 substances. 11 This bill would list salvia divinorum and 12 Salvinorin A as Schedule I controlled substances. 13 14 A BILL 15 TO BE ENTITLED 16 AN ACT 17 18 To amend Section 20-2-23 of the Code of Alabama 19 1975, relating to Schedule I controlled substances, to list 20 salvia divinorum and Salvinorin A. 21 BE IT ENACTED BY THE LEGISLATURE OF ALABAMA: 22 Section 1. Section 20-2-23 of the Code of Alabama 23 1975, is amended to read as follows: 24 "§20-2-23. 25 "The controlled substances listed in this section 26 are included in Schedule I: Page 1 1 "(1) Any of the following opiates, including their 2 isomers, esters, ethers, salts, and salts of isomers, esters 3 and ethers, unless specifically excepted, whenever the 4 existence of these isomers, esters, ethers and salts is 5 possible within the specific chemical designation: 6 "a. Acetylmethadol; 7 "b. Allylprodine; 8 "c. Alphacetylmethadol; 9 "d. Alphameprodine; 10 "e. Alphamethadol; 11 "f. Benzethidine; 12 "g. Betacetylmethadol; 13 "h. Betameprodine; 14 "i. Betamethadol; 15 "j. Betaprodine; 16 "k. Clonitazene; 17 "l. Dextromoramide; 18 "m. Dextrorphan; 19 "n. Diampromide; 20 "o. Diethylthiambutene; 21 "p. Dimenoxadol; 22 "q. Dimepheptanol; 23 "r. -

Guidelines for Ensuring Patient Access To, and Safe Management Of, Controlled Medicines Notice
African Palliative Care Association Guidelines for Ensuring Patient Access to, and Safe Management of, Controlled Medicines Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. APCA and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of this publication. However, in view of the possibility of human error or changes in sciences, neither APCA nor the publisher nor any other party who have been involved in the preparation or publication of this work warrants that the information contained in this publication is complete and correct and they disclaim all responsibility for any errors or omission or for the results obtained from use of the information contained herein. African Palliative Care Association Foreword The 59th Session of the World Health conventions. Several countries make the Assembly of the UN adopted resolution importation, storage, distribution and 58.22, thereby recognising the dispensing of controlled medicines more importance of improving pain relief restrictive than is needed. Additionally, using opioid analgesics and calling on it is a requirement for countries to member states to remove barriers to provide detailed annual estimates and their medical use and availability. reports for narcotic substances to the International Narcotic Control Board to While advances have been made pursuing procure or produce controlled medicines. this agenda in Africa (e.g. legalisation of However, formulating reliable estimates oral morphine prescription rights for nurses is often a barrier to accessing controlled and clinical officers in Uganda, approval medicines, while the procurement of access to morphine for hospices of opioids is subject to complex and in Zambia, and advocacy progress in lengthy exportation and importation Malawi and Kenya), challenges remain. -

Narcotic Drugs and Psychotropic Substances (Control) Act
LAWS OF GUYANA Narcotic Drugs and Psychotropic Cap. 35:11 3 Substances (Control) CHAPTER 35:11 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONTROL) ACT ARRANGEMENT OF SECTIONS PART I PRELIMINARY SECTION 1. Short title. 2. Interpretation. PART II PROHIBITION OF POSSESSION OF AND TRAFFICKING IN NARCOTICS AND CULTIVATION OF CERTAIN PLANTS 3. Definitions for Part II. 4. Penalty for possession of narcotic. 5. Penalty for trafficking in narcotic. 6. Penalty for supply, etc., of narcotic to child or young person if death results from consumption or administration of it. 7. Penalty for bringing into prison or taking out of prison, etc., of a narcotic. 8. Penalty for cultivation of certain plants. 9. Procedure for purposes of section 8(2) to (6). 10. Power of entry in respect of State or Government lands. 11. Power of destruction of prohibited plants. 12. Penalty for certain other acts connected with narcotics. 13. Certain prescriptions to be unlawful. 14. Penalty for receiving additional narcotic or prescription without disclosure of earlier receipt. 15. Removal of name from register. L.R.O. 3/1998 LAWS OF GUYANA 4 Cap. 35:11 Narcotic Drugs and Psychotropic Substances (Control) PART III NARCOTICS IN TRANSIT SECTION 16. Definitions for Part III. 17. Prohibition against sending narcotics by post. 18. Narcotics in transit. 19. Control of Comptroller over narcotics brought into Guyana in transit. 20. Tampering with narcotics in transit. 21. Diversion in Guyana of narcotics in transit. 22. Variations in export authorisation, import authorisation or diversion certificate granted in country other than Guyana. PART IV LICENCES 23. Grant and renewal of licences. -
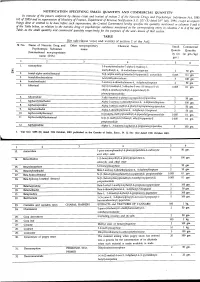
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

Outline for Controlled Substances Program
Environmental Health and Safety Controlled Substances Program Date of Issuance: Review Date: 10/1/2019 (no changes) 10/01/2018 Revision Number: Initial Prepared by: EH&S Table of Contents HEADINGS Introduction Applicability Responsibilities Registration Requirements Authorized Use Ordering/Purchasing Administering and Dispensing Inventory Procedures (Continuing Records) Security Disposal FORMS: Registering or renewing a DEA or state license (CMU) Controlled Substances Authorized users list (CMU) Employee questionnaire for those with access to controlled substances (CMU) Record of Form 222 use (Order form) (CMU) Records of Controlled Substance Purchases (CMU) Record of Controlled Substance Administering and dispensing (CMU) Controlled Substance Physical Inventory (CMU) DEA Registration of Persons doing research or analysis (Form 225) DEA Registration of Dispensers (Form 224) DEA Registration Instructional (Form 224 and 226 to renew) DEA Report of loss or theft (Form 106) DEA Report of drugs surrendered (From 41) DEA SCHEDULES: Schedule I Schedule II Schedule III Schedule IV Schedule V INTRODUCTION State and Federal regulations have been promulgated concerning the use and handling of US Department of Justice Drug Enforcement Administration (DEA) controlled substances. These regulations are in place to address materials which are or have the potential to be addictive or habit forming. These substances have been categorized into “schedules” that have been created by the DEA to reflect their level of concern. The “Carnegie Mellon University DEA Controlled Substances Program” is intended to ensure that Carnegie Mellon University is in compliance with our regulatory requirements. Required activities under the DEA include: 1. Registration of your work with the DEA and with Carnegie Mellon’s Department of Environmental Health and Safety (EH&S). -
![Chapter 329 [New] Uniform Controlled Substances Act](https://docslib.b-cdn.net/cover/1442/chapter-329-new-uniform-controlled-substances-act-1221442.webp)
Chapter 329 [New] Uniform Controlled Substances Act
CHAPTER 329 [NEW] UNIFORM CONTROLLED SUBSTANCES ACT Part I. General Provisions Section 329-1 Definitions 329-2 Hawaii advisory commission on drug abuse and controlled substances; number; appointment 329-3 Annual report 329-4 Duties of the commission Part II. Standards and Schedules 329-11 Authority to schedule controlled substances 329-12 Nomenclature 329-13 Schedule I tests 329-14 Schedule I 329-15 Schedule II tests 329-16 Schedule II 329-17 Schedule III Tests 329-18 Schedule III 329-19 Schedule IV tests 329-20 Schedule IV 329-21 Schedule V tests 329-22 Schedule V 329-23 Republishing and distribution of schedules Part III. Regulation of Manufacture, Distribution, Prescription, and Dispensing of Controlled Substances 329-31 Rules 329-31.5 Clinics 329-32 Registration requirements 329-33 Registration 329-34 Revocation and suspension of registration 329-35 Order to show cause 329-36 Records of registrants 329-37 Filing requirements 329-38 Prescriptions 329-39 Labels 329-40 Methadone treatment programs Part IV. Offenses and Penalties 329-41 Prohibited acts B-penalties 329-42 Prohibited acts C-penalties 329-43 Penalties under other laws 329-43.5 Prohibited acts related to drug paraphernalia Amended 0612 1 329-44 Notice of conviction to be sent to licensing board, department of commerce and consumer affairs 329-45 Repealed 329-46 Prohibited acts related to visits to more than one practitioner to obtain controlled substance prescriptions 329-49 Administrative penalties 329-50 Injunctive relief Part V. Enforcement and Administrative Provisions 329-51 Powers of enforcement personnel 329-52 Administrative inspections 329-53 Injunctions 329-54 Cooperative arrangements and confidentiality 329-55 Forfeitures 329-56 Burden of proof; liabilities 329-57 Judicial review 329-58 Education and research 329-59 Controlled substance registration revolving fund; established Part VI.