Guidelines for Ensuring Patient Access To, and Safe Management Of, Controlled Medicines Notice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening/Spot Test of Narcotics
Indian Journal of Forensic and Community Medicine 2020;7(4):160–165 Content available at: https://www.ipinnovative.com/open-access-journals Indian Journal of Forensic and Community Medicine Journal homepage: https://www.ipinnovative.com/journals/IJFCM Review Article Screening/spot test of narcotics A K Jaiswal1,*, Kamna Sharma2, Rohit Kanojia3, Sally Lukose4 1Dept. of Forensic Medicine & Toxicology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 2Galgotias University, Greater Noida, Uttar Pradesh, India 3Dept. of Chemistry, University of Delhi, New Delhi, India 4CTM-IRTE, Faridabad, Haryana, India ARTICLEINFO ABSTRACT Article history: Narcotics are the substances used to treat moderate to severe pain. They could be natural like opiates such Received 25-11-2020 as morphine, codeine etc., synthetic like fentanyl, methadone etc., and semi-synthetic like oxycodone, Accepted 02-12-2020 hydrocodone etc. These drugs act as pain relievers, induces the state of stupor or sleep, and increase Available online 08-01-2021 the physical dependence on them. In forensic autopsy case, the forensic pathologist may require a complete toxicological investigation for different poisons including stimulants. In India, Forensic Science Laboratories run by Government under the Home ministry usually carry out this. The samples must be Keywords: analysed by the forensic toxicologist/chemists/scientist. This article deals with the screening/spot test for Narcotics narcotics. It attempts to simplify the standard procedures in a step-wise manner, which can be of handy Screening reference for the forensic toxicologist. Spot test Drugs © This is an open access article distributed under the terms of the Creative Commons Attribution Opioids etc License (https://creativecommons.org/licenses/by/4.0/) which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0024006 A1 Simon (43) Pub
US 2004.0024006A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0024006 A1 Simon (43) Pub. Date: Feb. 5, 2004 (54) OPIOID PHARMACEUTICAL May 30, 1997, now abandoned, and which is a COMPOSITIONS continuation-in-part of application No. 08/643,775, filed on May 6, 1996, now abandoned. (76) Inventor: David Lew Simon, Mansfield Center, CT (US) Publication Classification Correspondence Address: (51) Int. Cl. ................................................ A61K 31/485 David L. Simon (52) U.S. Cl. .............................................................. 514/282 P.O. Box 618 100 Cemetery Road (57) ABSTRACT Mansfield Center, CT 06250 (US) The invention is directed in part to dosage forms comprising a combination of an analgesically effective amount of an (21) Appl. No.: 10/628,089 opioid agonist analgesic and a neutral receptor binding agent or a partial mu-opioid agonist, the neutral receptor binding (22) Filed: Jul. 25, 2003 agent or partial mu-opioid agonist being included in a ratio Related U.S. Application Data to the opioid agonist analgesic to provide a combination product which is analgesically effective when the combina (63) Continuation-in-part of application No. 10/306,657, tion is administered as prescribed, but which is leSS analge filed on Nov. 27, 2002, which is a continuation-in-part Sically effective or less rewarding when administered in of application No. 09/922,873, filed on Aug. 6, 2001, excess of prescription. Preferably, the combination product now Pat. No. 6,569,866, which is a continuation-in affects an opioid dependent individual differently from an part of application No. 09/152,834, filed on Sep. -

Introduced B.,Byhansen, 16
LB301 LB301 2021 2021 LEGISLATURE OF NEBRASKA ONE HUNDRED SEVENTH LEGISLATURE FIRST SESSION LEGISLATIVE BILL 301 Introduced by Hansen, B., 16. Read first time January 12, 2021 Committee: Judiciary 1 A BILL FOR AN ACT relating to the Uniform Controlled Substances Act; to 2 amend sections 28-401, 28-405, and 28-416, Revised Statutes 3 Cumulative Supplement, 2020; to redefine terms; to change drug 4 schedules and adopt federal drug provisions; to change a penalty 5 provision; and to repeal the original sections. 6 Be it enacted by the people of the State of Nebraska, -1- LB301 LB301 2021 2021 1 Section 1. Section 28-401, Revised Statutes Cumulative Supplement, 2 2020, is amended to read: 3 28-401 As used in the Uniform Controlled Substances Act, unless the 4 context otherwise requires: 5 (1) Administer means to directly apply a controlled substance by 6 injection, inhalation, ingestion, or any other means to the body of a 7 patient or research subject; 8 (2) Agent means an authorized person who acts on behalf of or at the 9 direction of another person but does not include a common or contract 10 carrier, public warehouse keeper, or employee of a carrier or warehouse 11 keeper; 12 (3) Administration means the Drug Enforcement Administration of the 13 United States Department of Justice; 14 (4) Controlled substance means a drug, biological, substance, or 15 immediate precursor in Schedules I through V of section 28-405. 16 Controlled substance does not include distilled spirits, wine, malt 17 beverages, tobacco, hemp, or any nonnarcotic substance if such substance 18 may, under the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. -

Narcotic Drugs and Psychotropic Substances (Control) Act
LAWS OF GUYANA Narcotic Drugs and Psychotropic Cap. 35:11 3 Substances (Control) CHAPTER 35:11 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONTROL) ACT ARRANGEMENT OF SECTIONS PART I PRELIMINARY SECTION 1. Short title. 2. Interpretation. PART II PROHIBITION OF POSSESSION OF AND TRAFFICKING IN NARCOTICS AND CULTIVATION OF CERTAIN PLANTS 3. Definitions for Part II. 4. Penalty for possession of narcotic. 5. Penalty for trafficking in narcotic. 6. Penalty for supply, etc., of narcotic to child or young person if death results from consumption or administration of it. 7. Penalty for bringing into prison or taking out of prison, etc., of a narcotic. 8. Penalty for cultivation of certain plants. 9. Procedure for purposes of section 8(2) to (6). 10. Power of entry in respect of State or Government lands. 11. Power of destruction of prohibited plants. 12. Penalty for certain other acts connected with narcotics. 13. Certain prescriptions to be unlawful. 14. Penalty for receiving additional narcotic or prescription without disclosure of earlier receipt. 15. Removal of name from register. L.R.O. 3/1998 LAWS OF GUYANA 4 Cap. 35:11 Narcotic Drugs and Psychotropic Substances (Control) PART III NARCOTICS IN TRANSIT SECTION 16. Definitions for Part III. 17. Prohibition against sending narcotics by post. 18. Narcotics in transit. 19. Control of Comptroller over narcotics brought into Guyana in transit. 20. Tampering with narcotics in transit. 21. Diversion in Guyana of narcotics in transit. 22. Variations in export authorisation, import authorisation or diversion certificate granted in country other than Guyana. PART IV LICENCES 23. Grant and renewal of licences. -
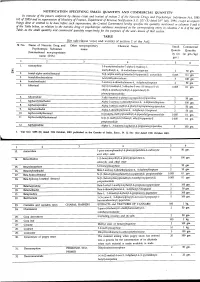
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

The International Drug Control Conventions
ST/CND/1/Add.1/Rev.3 The International Drug Control Conventions Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 UNITED NATIONS New York, 2017 ST/CND/1/Add.1/Rev.3 © United Nations, 2017. All rights reserved, worldwide. Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 List of drugs included in Schedule I Acetorphine 3-O-Acetyltetrahydro-7α-(1-hydroxy-1-methylbutyl)- 6,14-endo-ethenooripavine Acetyl-alpha-methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]acetanilide Acetylfentanyl N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]acetamide Acetylmethadol 3-Acetoxy-6-dimethylamino-4,4-diphenylheptane AH-7921 3,4-dichloro-N-{[1- (dimethylamino)cyclohexyl]methyl}benzamide Alfentanil N-[1-[2-(4-Ethyl-4,5-dihydro-5-oxo-1H-tetrazol-1-yl) ethyl]-4-(methoxymethyl)-4-piperidinyl]-N- phenylpropanamide Allylprodine 3-Allyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphacetylmethadol α-3-Acetoxy-6-dimethylamino-4,4-diphenylheptane Alphameprodine α-3-Ethyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphamethadol α-6-Dimethylamino-4,4-diphenyl-3-heptanol alpha-Methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]propionanilide alpha-Methylthiofentanyl N-[1-[1-Methyl-2-(2-thienyl)ethyl]-4-piperidyl] propionanilide Alphaprodine α-l,3-Dimethyl-4-phenyl-4-propionoxypiperidine Anileridine 1-p-Aminophenethyl-4-phenylpiperidine-4-carboxylic acid ethyl ester Benzethidine 1-(2-Benzyloxyethyl)-4-phenylpiperidine-4-carboxylic acid ethyl ester -

The Prevention and Treatment of Drug Misuse in Britain
If you have issues viewing or accessing this file contact us at NCJRS.gov. .. " FW The prevention and treatment of drug misuse in Britain "I Issued by Rererence Division BRITISH INFORMATION SERVICES All Agency of the British Government 845 THIRD AVENUE, NEW YORK, N.Y. ]0022 This material is prepared, edited, issued or circulated by British Information Servic(!s, 845 Third Avenue, New York, N. Y.l0022, which is registered under the Foreign Agents Registration Act as an agent of the Brilish Government. This material is filed with the Department of Justice where the required registration statement is available for public inspection. Registration does not indicate approval 0/ the contents of this material by the United States Government. PRINTED IN ENGLAND BY TRADE UNION LABOR BY COLUNS AND WILSON LTD., ANDOVER The prevention and treatment of drug misuse in Britain Prepared by REFERENCE DIVISION CENTRhL OFFICE OF INFORMATION, LONDON Oclo~'er 1978 Quote No RS94S/78 CLASSIFICATION 4(c) N.B.-This pamphlet is i//tellded to be used for referellce purposes alld may be freely used ill preparing articles, speeches, broadcasts, elc. No acknowledgment is necessary. Please Ilote the date ofpreparation. Tire text gives general gUidance only, and should 1I0t be treated as an authoritative statement of the law. Pamphlets ill this series may be obtained from the Tllformation Office at tlte British Embassy, Consulate or High Commission in tile inquirer's Co/lIltl'Y of residellce. CONTENTS Page INTRODUCTION 1 BACKGROUND .. 3 PREVENTING DRUG MISUSE 8 The Law 8 Health Education 17 TREATMENT AND REHABILITATION .. 20 Narcotic Drug Misuse and the Role of the Clinics 20 Treating Other Forms of Drug Misuse 26 Rehabilitation 26 INFORMATION AND RESEARCH ., 29 APPENDICES 1. -

Supplement 1: Additional Tables and Figures
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplement 1: Additional tables and figures Box S1: Substances included and excluded from the International Narcotic Control Board (INCB) data on narcotic consumption, in alphabetical order. Opioids included in the opioid consumption calculation: 1. (+)-cis-3-methylfental 35. Bezitramide 2. 3-Acetylmorphine 36. Butyrfentanyl 3. 3-Methylfentanyl 37. Carfentanil 4. 3-Methylthiofentanyl 38. Carfentanyl 5. 3-Monoacetylmorphine 39. Clonitazene 6. 4-Fluoroisobutyrfentanyl 40. Codeine 7. 6-Acetylmorphine 41. Codeine-6GLUC 8. 6-Monoacetylmorphine 42. Codeine-6-glucuronide 9. Acetorphine 43. Codeine-Methyl 10. Acetyl-alpha-methylfentanyl 44. Codeine-N-oxide 11. Acetyldihydrocodeine 45. Codoxime 12. Acetylfentanyl 46. Conc. of poppy straw (C) ACA 13. Acetylmethadol 47. Conc. of poppy straw (C) AMA 14. Acetylmorphine 48. Conc. of poppy straw (C) AOA 15. Acrylfentanyl 49. Conc. of poppy straw (C) ATA 16. AH-7921 50. Conc. of poppy straw (C) GW 17. Alfentanil 51. Conc. of poppy straw (M) ACA 18. Allylprodine 52. Conc. of poppy straw (M) AMA 19. Alphacetylmethadol 53. Conc. of poppy straw (M) AOA 20. Alphameprodine 54. Conc. of poppy straw (M) ATA 21. Alphamethadol 55. Conc. of poppy straw (M) GW 22. alpha-Methylfentanyl 56. Conc. of poppy straw (N) GW 23. alpha-Methylthiofentanyl 57. Conc. of poppy straw (O) 24. Alphaprodine 58. Conc. of poppy straw (O) ACA 25. Anileridine 59. Conc. of poppy straw (O) AMA 26. Benzethidine 60. Conc. of poppy straw (O) AOA 27. -

List of Narcotic Drugs Under International Control
International Narcotics Control Board Yellow List Annex to Forms A, B and C 59th edition, July 2020 LIST OF NARCOTIC DRUGS UNDER INTERNATIONAL CONTROL Prepared by the INTERNATIONAL NARCOTICS CONTROL BOARD* Vienna International Centre P.O. Box 500 A-1400 Vienna, Austria Internet address: http://www.incb.org/ in accordance with the Single Convention on Narcotic Drugs, 1961** Protocol of 25 March 1972 amending the Single Convention on Narcotic Drugs, 1961 * On 2 March 1968, this organ took over the functions of the Permanent Central Narcotics Board and the Drug Supervisory Body, r etaining the same secretariat and offices. ** Subsequently referred to as “1961 Convention”. V.20-03697 (E) *2003697* Purpose The Yellow List contains the current list of narcotic drugs under international control and additional relevant information. It has been prepared by the International Narcotics Control Board to assist Governments in completing the annual statistical reports on narcotic drugs (Form C), the quarterly statistics of imports and exports of narcotic drugs (Form A) and the estimates of annual requirements for narcotic drugs (Form B) as well as related questionnaires. The Yellow List is divided into four parts: Part 1 provides a list of narcotic drugs under international control in the form of tables and is subdivided into three sections: (1) the first section includes the narcotic drugs listed in Schedule I of the 1961 Convention as well as intermediate opiate raw materials; (2) the second section includes the narcotic drugs listed in Schedule II of the 1961 Convention; and (3) the third section includes the narcotic drugs listed in Schedule IV of the 1961 Convention. -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: