NAC/AEGL Committee Meeting, April 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Air Contaminants – Permissible Exposure Limits (Pels)
SUBPART Z -- TOXIC AND HAZARDOUS SUBSTANCES 1910.1000-AIR CONTAMINANTS An employee’s exposure to any substance listed in Table Z-1-A of this section shall be limited in accordance with the requirements of the following paragraphs of this section. (a) Table Z-1-A. Limits for Air Contaminants (1) & (2) Enforcement of Transitional Limits has expired. See Paragraph (3) for Limits. (3) Limits for Air Contaminants Columns. An employee’s exposure to any substance listed in Table Z-1-A shall not exceed the Time Weighted Average (TWA), Short Term Exposure Limit (STEL) and Ceiling Limit specified for that substance in Table Z-1-A. (4) Skin Designation. To prevent or reduce skin absorption, an employee’s skin exposure to substances listed in Table Z-1-A with an “X” in the Skin Designation column following the substance name shall be prevented or reduced to the extent necessary in the circumstances through the use of gloves, coveralls, goggles, or other appropriate personal protective equipment, engineering controls or work practices. (5) Definitions. The following definitions are applicable to the Limits for Air Contaminants columns of Table Z- 1-A: (i) Time weighted average (TWA) is the employee’s average airborne exposure in any 8-hour work shift of a 40-hour work week which shall not be exceeded. (ii) Short term exposure limit (STEL) is the employee’s 15-minute time weighted average exposure which shall not be exceeded at any time during a work day unless another time limit is specified in a parenthetical notation below the limit. -

Rhode Island Hazardous Substance List
Rhode Island Hazardous Substance List Source: T - ACGIH F - NFPA49 C - IARC Alphabetical Order C.A.S. ACGIH NFPA IARC CHEMICAL NAME 13010-47-4 C 1,-(2-Chloroethyl)-3-cyclohexyl-1-Nitrosourea 76-11-9 T 1,1,1,2-tetrachloro-2,2-difluoroethane 76-12-0 T 1,1,2,2-tetrachloro-1,2-difluoroethane 79-34-5 T 1,1,2,2-tetrachloroethane - skin 76-13-1 T 1,1,2-trichloro-1,2,2-trifluoroethane 79-00-5 T F C 1,1,2-trichloroethane - skin 594-72-9 T 1,1-Dichloro-1-nitroethane 74-34-3 T 1,1-dichloroethane 57-14-7 T 1,1-dimethylhydrazine (udmh) 96-18-4 T 1,2,3-trichloropropane 120-82-1 T 1,2,4-Trichlorobenzene 106-88-7 F 1,2-Butylene oxide 107-15-3 T F 1,2-Diaminoethane 96-12-8 C 1,2-Dibromo-3-chloropropane 106-93-4 T F C 1,2-Dibromoethane - skin 107-06-2 T F 1,2-Dichlorethane 540-59-0 T F 1,2-Dichloroethene 540-59-0 T F 1,2-Dichloroetylene 1615-80-1 C 1,2-Diethylhydrazine C 1,2-Dimethyl hydrazine - skin 106-99-0 T F 1,3-Butadiene 118-52-5 T 1,3-Dichloro-5,5-dimethylhydantoin 542-75-6 T F 1,3-Dichloropropene (cis and trans) 542-75-6 T F 1,3-Dichloropropylene 110-56-5 F 1,4-Dichlorobutane 123-91-1 T F C 1,4-Dioxane 1120-71-4 1-3-Propane sultone 110-53-2 F 1-Bromopentane 106-89-8 T F C 1-Chloro,2,3-epoxy-propane 600-25-9 T 1-Chloro-1-nitropropane 97-00-7 F 1-chloro-2,4-dinitrobenzene 543-59-9 F 1-Chloropentane 112-30-1 F 1-Decanol 111-27-3 F 1-Hexanol 141-79-7 T F 1-Isobutenyl methyl ketone 108-03-2 T F 1-Nitropropane 71-41-0 F 1-Pentanol 110-58-7 F 1-Pentylamine 111-40-0 T F 2,2'-Diaminodiethylamine 111-44-4 F 2,2'Dichlorodiethyl ether 75-99-0 T 2,2-dichloropropionic acid 556-52-5 T 2,3-Epoxy-1-propanol 93-76-5 T 2,4,5-T 95-95-4 F 2,4,5-trichlorophenol 88-06-2 F C 2,4,6-trichlorophenol 118-96-7 T F 2,4,6-Trinitro Toluene 479-95-8 T 2,4,6-Trinitrophenyl-methylnitramine 94-75-7 T 2,4-d (2,4-dichlorophenoxyacetic acid) 97-02-9 F 2,4-dinitroaniline 584-84-9 T F 2,4-Tolylene diisocyanate 108-83-8 T 2,6-Dimethyl-4-heptanone 108-83-8 T 2,6-Dimethyl-4-heptanone 128-37-0 T 2,6-Ditert. -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Gas Conversion Factor for 300 Series
300GasTable Rec # Gas Symbol GCF Density (g/L) Density (g/L) 25° C / 1 atm 0° C / 1 atm 1 Acetic Acid C2H4F2 0.4155 2.7 2.947 2 Acetic Anhydride C4H6O3 0.258 4.173 4.555 3 Acetone C3H6O 0.3556 2.374 2.591 4 Acetonitryl C2H3N 0.5178 1.678 1.832 5 Acetylene C2H2 0.6255 1.064 1.162 6 Air Air 1.0015 1.185 1.293 7 Allene C3H4 0.4514 1.638 1.787 8 Ammonia NH3 0.7807 0.696 0.76 9 Argon Ar 1.4047 1.633 1.782 10 Arsine AsH3 0.7592 3.186 3.478 11 Benzene C6H6 0.3057 3.193 3.485 12 Boron Trichloride BCl3 0.4421 4.789 5.228 13 Boron Triflouride BF3 0.5431 2.772 3.025 14 Bromine Br2 0.8007 6.532 7.13 15 Bromochlorodifluoromethane CBrClF2 0.3684 6.759 7.378 16 Bromodifluoromethane CHBrF2 0.4644 5.351 5.841 17 Bromotrifluormethane CBrF3 0.3943 6.087 6.644 18 Butane C4H10 0.2622 2.376 2.593 19 Butanol C4H10O 0.2406 3.03 3.307 20 Butene C4H8 0.3056 2.293 2.503 21 Carbon Dioxide CO2 0.7526 1.799 1.964 22 Carbon Disulfide CS2 0.616 3.112 3.397 23 Carbon Monoxide CO 1.0012 1.145 1.25 24 Carbon Tetrachloride CCl4 0.3333 6.287 6.863 25 Carbonyl Sulfide COS 0.668 2.456 2.68 26 Chlorine Cl2 0.8451 2.898 3.163 27 Chlorine Trifluoride ClF3 0.4496 3.779 4.125 28 Chlorobenzene C6H5Cl 0.2614 4.601 5.022 29 Chlorodifluoroethane C2H3ClF2 0.3216 4.108 4.484 30 Chloroform CHCl3 0.4192 4.879 5.326 31 Chloropentafluoroethane C2ClF5 0.2437 6.314 6.892 32 Chloropropane C3H7Cl 0.308 3.21 3.504 33 Cisbutene C4H8 0.3004 2.293 2.503 34 Cyanogen C2N2 0.4924 2.127 2.322 35 Cyanogen Chloride ClCN 0.6486 2.513 2.743 36 Cyclobutane C4H8 0.3562 2.293 2.503 37 Cyclopropane C3H6 0.4562 -

The Hydrolysis of Phosphinates and Phosphonates: a Review
molecules Review The Hydrolysis of Phosphinates and Phosphonates: A Review Nikoletta Harsági and György Keglevich * Department of Organic Chemistry and Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary; [email protected] * Correspondence: [email protected]; Tel.: +36-1-463-1111 (ext. 5883) Abstract: Phosphinic and phosphonic acids are useful intermediates and biologically active com- pounds which may be prepared from their esters, phosphinates and phosphonates, respectively, by hydrolysis or dealkylation. The hydrolysis may take place both under acidic and basic conditions, but the C-O bond may also be cleaved by trimethylsilyl halides. The hydrolysis of P-esters is a challenging task because, in most cases, the optimized reaction conditions have not yet been explored. Despite the importance of the hydrolysis of P-esters, this field has not yet been fully surveyed. In order to fill this gap, examples of acidic and alkaline hydrolysis, as well as the dealkylation of phosphinates and phosphonates, are summarized in this review. Keywords: hydrolysis; dealkylation; phosphinates; phosphonates; P-acids 1. Introduction Phosphinic and phosphonic acids are of great importance due to their biological activity (Figure1)[ 1]. Most of them are known as antibacterial agents [2,3]. Multidrug- resistant (MDR) and extensively drug-resistant (XDR) pathogens may cause major problems Citation: Harsági, N.; Keglevich, G. in the treatment of bacterial infections. However, Fosfomycin has remained active against The Hydrolysis of Phosphinates and both Gram-positive and Gram-negative MDR and XDR bacteria [2]. Acyclic nucleoside Phosphonates: A Review. Molecules phosphonic derivatives like Cidofovir, Adefovir and Tenofovir play an important role 2021, 26, 2840. -

Perchloryl Fluoride Final AEGL
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=15852 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-29025-8 Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council 292 pages 6 x 9 PAPERBACK (2013) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. -
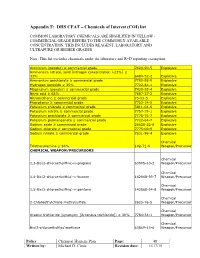
Appendix F: DHS CFAT – Chemicals of Interest (COI) List
Appendix F: DHS CFAT – Chemicals of Interest (COI) list COMMON LABORATORY CHEMICALS ARE HIGHLITED IN YELLOW - COMMERCIAL GRADE REFERS TO THE COMMONLY AVAILABLE CONCENTRATION, THIS INCLUDES REAGENT, LABORATORY AND ULTRAPURE OR HIGHER GRADES Note: This list excludes chemicals under the laboratory and R+D reporting exemption. Aluminum (powder) ≥ commercial grade 7429-90-5 Explosive Ammonium nitrate, solid [nitrogen concentration >23%] ≥ 33% 6484-52-2 Explosive Ammonium perchlorate ≥ commercial grade 7790-98-9 Explosive Hydrogen peroxide ≥ 35% 7722-84-1 Explosive Magnesium (powder) ≥ commercial grade 7439-95-4 Explosive Nitric acid ≥ 68% 7697-37-2 Explosive Nitromethane ≥ commercial grade 75-52-5 Explosive Phosphorus ≥ commercial grade 7723-14-0 Explosive Potassium chlorate ≥ commercial grade 3811-04-9 Explosive Potassium nitrate ≥ commercial grade 7757-79-1 Explosive Potassium perchlorate ≥ commercial grade 7778-74-7 Explosive Potassium permanganate ≥ commercial grade 7722-64-7 Explosive Sodium azide ≥ commercial grade 26628-22-8 Explosive Sodium chlorate ≥ commercial grade 7775-09-9 Explosive Sodium nitrate ≥ commercial grade 7631-99-4 Explosive Chemical Triethanolamine ≥ 80% 102-71-6 Weapon/Precursor CHEMICAL WEAPON/PRECURSORS Chemical 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 Weapon/Precursor Chemical 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 Weapon/Precursor Chemical 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 Weapon/Precursor Chemical 2-Chloroethylchloro-methylsulfide 2625-76-5 Weapon/Precursor Chemical Arsenic trichloride -

TIH/PIH List
Hazardous Materials Designated as TIH/PIH (consolidated AAR and Railinc lists) 3/12/2007 STCC Proper Shipping Name 4921402 2-CHLOROETHANAL 4921495 2-METHYL-2-HEPTANETHIOL 4921741 3,5-DICHLORO-2,4,6-TRIFLUOROPYRIDINE 4921401 ACETONE CYANOHYDRIN, STABILIZED 4927007 ACROLEIN, STABILIZED 4921019 ALLYL ALCOHOL 4923113 ALLYL CHLOROFORMATE 4921004 ALLYLAMINE 4904211 AMMONIA SOLUTION 4920360 AMMONIA SOLUTIONS 4904209 AMMONIA, ANHYDROUS 4904210 AMMONIA, ANHYDROUS 4904879 AMMONIA, ANHYDROUS 4920359 AMMONIA, ANHYDROUS 4923209 ARSENIC TRICHLORIDE 4920135 ARSINE 4932010 BORON TRIBROMIDE 4920349 BORON TRICHLORIDE 4920522 BORON TRIFLUORIDE 4936110 BROMINE 4920715 BROMINE CHLORIDE 4918505 BROMINE PENTAFLUORIDE 4936106 BROMINE SOLUTIONS 4918507 BROMINE TRIFLUORIDE 4921727 BROMOACETONE 4920343 CARBON MONOXIDE AND HYDROGEN MIXTURE, COMPRESSED 4920399 CARBON MONOXIDE, COMPRESSED 4920511 CARBON MONOXIDE, REFRIGERATED LIQUID 4920559 CARBONYL FLUORIDE 4920351 CARBONYL SULFIDE 4920523 CHLORINE 4920189 CHLORINE PENTAFLUORIDE 4920352 CHLORINE TRIFLUORIDE 4921558 CHLOROACETONE, STABILIZED 4921009 CHLOROACETONITRILE 4923117 CHLOROACETYL CHLORIDE 4921414 CHLOROPICRIN 4920516 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920547 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920392 CHLOROPICRIN AND METHYL CHLORIDE MIXTURES 4921746 CHLOROPIVALOYL CHLORIDE 4930204 CHLOROSULFONIC ACID 4920527 COAL GAS, COMPRESSED 4920102 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920303 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920304 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920305 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920101 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920300 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920301 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920324 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920331 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920165 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920378 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920379 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. -

Chemicals Requiring EHS Pre-Approval
Chemicals Requiring EHS Approval Before Purchasing Chemical Name CAS # 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 1H-Tetrazole 288-94-8 2-chloroethyl ethylsulfide 693-07-2 2-Chloroethylchloro-methylsulfide 2625-76-5 2-Cyanoethyl diisopropylchlorophosphoramidite 89992-70-1 2-Ethoxyethanol 110-80-5 2-Ethoxyethylacetate 111-15-9 2-Methoxyethanol 109-86-4 2-Methoxyethylacetate 110-49-6 5-Nitrobenzotriazol 2338-12-7 Acetone cyanohydrin, stabilized 75-86-5 Acrolein 107-02-8 Acrylamide (powder) 79-06-1 Allylamine 107-11-9 Aluminum (powder) 7429-90-5 Aluminum phosphide 20859-73-8 Ammonia 7664-41-7 Ammonium nitrate 6484-52-2 Ammonium perchlorate 7790-98-9 Ammonium picrate 131-74-8 Arsenic 7440-38-2 Arsenic trichloride 7784-34-1 Arsenic trioxide 1327-53-3 Arsine 7784-42-1 Barium azide 18810-58-7 Beryllium 7440-41-7 Bis(2-chloroethylthio)methane 63869-13-6 Bis(2-chloroethylthiomethyl)ether 63918-90-1 Boron tribromide 10294-33-4 Boron trichloride 10294-34-5 Boron trifluoride 7637-07-2 Bromine 7726-95-6 Bromine chloride 13863-41-7 Bromine pentafluoride 7789-30-2 Bromine trifluoride 7787-71-5 Cadmium 7440-43-9 Calcium phosphide 1305-99-3 Carbon monoxide 630-08-0 Carbonyl fluoride 353-50-4 Carbonyl sulfide 463-58-1 Chlorine 7782-50-5 Chlorine dioxide 10049-04-4 Chlorine pentafluoride 13637-63-3 Chlorine trifluoride 7790-91-2 Chemicals Requiring EHS Approval Before Purchasing Chloroacetyl chloride 79-04-9 Chlorosarin 1445-76-7 Chlorosoman 7040-57-5 Chlorosulfonic -

Appendix a to Part 27
Appendix A to Part 27. – DHS Chemicals of Interest1 - - - WME - - – – Chemical Chemicals of Interest Abstract Synonym (COI) Service (CAS) # y Issue: Theft Theft y Issue: Screening Threshold Threshold Screening EXP/IEDP Issue: Security Sabotage/Contamination Security Issue: Theft Theft Issue: Security CWI/CWP Theft Issue: Security Release: Minimum Minimum Release: (%) Concentration Screening Release: (in Quantities Threshold pounds) Theft: Minimum (%) Concentration Theft: pounds (in Quantities noted) otherwise unless Minimum Sabotage: (%) Concentration Screening Sabotage: Quantities Threshold Release Issue: Security Toxic Release Issue: Security Flammables Release Issue: Security Explosives Securit Acetaldehyde 75-07-0 1.00 10,000 X Acetone cyanohydrin, 75-86-5 ACG APA X stabilized Acetyl bromide 506-96-7 ACG APA X Acetyl chloride 75-36-5 ACG APA X Acetyl iodine 507-02-8 ACG APA X Acetylene [Ethyne] 74-86-2 1.00 10,000 X Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1.00 5,000 X Acrylonitrile [2-Propenenitrile] 107-13-1 1.00 10,000 X Acrylyl chloride [2-Propenoyl Chloride] 814-68-6 1.00 10,000 X Allyl alcohol [2-Propen-1-ol] 107-18-6 1.00 15,000 X Allylamine [2-Propen-1-amine] 107-11-9 1.00 10,000 X Allyltrichlorosilane, 107-37-9 ACG APA X stabilized Aluminum (powder) 7429-90-5 ACG 100 X Aluminum bromide, 7727-15-3 ACG APA X anhydrous Aluminum chloride, 7446-70-0 ACG APA X anhydrous Aluminum phosphide 20859-73-8 ACG APA X Ammonia (anhydrous) 7664-41-7 1.00 10,000 X 1 The acronyms used in this appendix have the following meaning: ACG