What Moths Fly in Winter? the Assemblage of Moths Active in a Temperate Deciduous Forest During the Cold Season in Central Poland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1.2 RP TVB ANNEXE ABT Especes Et Enjeux
La connaissance de la biodiversité sur l’Est Cantal un enjeu pour le développement du territoire ! Depuis avril 2017, le SYTEC s’est engagé dans une démarche d’Atlas de la Biodiversité Territoriale, avec l’objectif d’une part, de mieux connaitre la biodiversité du territoire et, d’autre part, d’intégrer les enjeux connus dans les démarches de planification notamment. Dans ce cadre, un ensemble de données naturalistes sur les territoires de l’Est Cantal ont pu être collectées auprès de nombreuses structures (Muséum National d’Histoire Naturelle, collectivités, associations, naturalistes professionnels…). Des inventaires sont également confiés chaque année par le SYTEC à des structures professionnelles pour compléter les connaissances sur des secteurs mal connus. En complément, des enquêtes participatives, qui rencontrent un vif succès, ont été lancées à destination du grand public, particulièrement des habitants des territoires. Ce travail de collecte a permis de réunir plus d’un million de données depuis le début de la démarche. A la fin de l’année 2018, ce sont 2505 espèces qui ont été répertoriés sur le territoire depuis 10 ans pour la faune (1017 espèces) et au cours de ces 15 dernières années pour la flore, les champignons et les algues (respectivement 1359, 31 et 98 espèces recensés). Parmi elles, 303 espèces sont considérées comme des espèces à enjeux en termes de préservation. Les inventaires se sont poursuivis en 2019 et continueront en 2020, permettant une mise à jour annuelle des connaissances de l’Est Cantal. Afin de partager -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Seasonal Changes in Lipid and Fatty Acid Profiles of Sakarya
Eurasian Journal of Forest Science ISSN: 2147 - 7493 Copyrights Eurasscience Journals Editor in Chief Hüseyin Barış TECİMEN University of Istanbul, Faculty of Forestry, Soil Science and Ecology Dept. İstanbul, Türkiye Journal Cover Design Mert EKŞİ Istanbul University Faculty of Forestry Department of Landscape Techniques Bahçeköy-Istanbul, Turkey Technical Advisory Osman Yalçın YILMAZ Surveying and Cadastre Department of Forestry Faculty of Istanbul University, 34473, Bahçeköy, Istanbul-Türkiye Cover Page Bolu forests, Turkey 2019 Ufuk COŞGUN Contact H. Barış TECİMEN Istanbul University-Cerrahpasa, Faculty of Forestry, Soil Science and Ecology Dept. İstanbul, Turkey [email protected] Journal Web Page http://dergipark.gov.tr/ejejfs Eurasian Journal of Forest Science Eurasian Journal of Forest Science is published 3 times per year in the electronic media. This journal provides immediate open access to its content on the principle that making research freely available to the public supports a greater global exchange of knowledge. In submitting the manuscript, the authors certify that: They are authorized by their coauthors to enter into these arrangements. The work described has not been published before (except in the form of an abstract or as part of a published lecture, review or thesis), that it is not under consideration for publication elsewhere, that its publication has been approved by all the authors and by the responsible authorities tacitly or explicitly of the institutes where the work has been carried out. They secure the right to reproduce any material that has already been published or copyrighted elsewhere. The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party. -

104612 Sletvoldtrunschkewimm
1 Separating selection by diurnal and nocturnal pollinators on floral display and spur length 2 in Gymnadenia conopsea 3 4 Nina Sletvold1,2, Judith Trunschke1,3, Carolina Wimmergren1, Jon Ågren1 5 6 1Plant Ecology and Evolution, Department of Ecology and Genetics, Evolutionary Biology 7 Centre, Uppsala University, Norbyvägen 18 D, SE-752 36 Uppsala, Sweden 8 E-mail: [email protected] 9 Phone: +46 18 471 28 71, Fax: +46 18 55 34 19 10 11 2Norwegian University of Science and Technology, Museum of Natural History and 12 Archaeology, N-7491 Trondheim, Norway 13 14 3Present address: Institute of Systematic Botany, University of Zurich, Zollikerstrasse 107, 15 CH-8008 Zürich, Switzerland 16 1 17 Abstract 18 Most plants attract multiple flower visitors that may vary widely in their effectiveness as 19 pollinators. Floral evolution is expected to reflect interactions with the most important 20 pollinators, but few studies have quantified the contribution of different pollinators to current 21 selection on floral traits. To compare selection mediated by diurnal and nocturnal pollinators on 22 floral display and spur length in the rewarding orchid Gymnadenia conopsea, we manipulated 23 the environment by conducting supplemental hand-pollinations and selective pollinator 24 exclusions in two populations in central Norway. In both populations, the exclusion of diurnal 25 pollinators significantly reduced seed production compared to open-pollination, whereas the 26 exclusion of nocturnal pollinators did not. There was significant selection on traits expected to 27 influence pollinator attraction and pollination efficiency in both the diurnal and nocturnal 28 pollination treatment. The relative strength of selection among plants exposed to diurnal and 29 nocturnal visitors varied among traits and populations, but the direction of selection was 30 consistent. -

Download (236Kb)
Chapter (non-refereed) Welch, R.C.. 1981 Insects on exotic broadleaved trees of the Fagaceae, namely Quercus borealis and species of Nothofagus. In: Last, F.T.; Gardiner, A.S., (eds.) Forest and woodland ecology: an account of research being done in ITE. Cambridge, NERC/Institute of Terrestrial Ecology, 110-115. (ITE Symposium, 8). Copyright © 1981 NERC This version available at http://nora.nerc.ac.uk/7059/ NERC has developed NORA to enable users to access research outputs wholly or partially funded by NERC. Copyright and other rights for material on this site are retained by the authors and/or other rights owners. Users should read the terms and conditions of use of this material at http://nora.nerc.ac.uk/policies.html#access This document is extracted from the publisher’s version of the volume. If you wish to cite this item please use the reference above or cite the NORA entry Contact CEH NORA team at [email protected] 110 The fauna, including pests, of woodlands and forests 25. INSECTS ON EXOTIC BROADLEAVED jeopardize Q. borealis here. Moeller (1967) suggest- TREES OF THE FAGACEAE, NAMELY ed that the general immunity of Q. borealis to QUERCUS BOREALIS AND SPECIES OF insect attack in Germany was due to its planting in NOTHOFAGUS mixtures with other Quercus spp. However, its defoliation by Tortrix viridana (green oak-roller) was observed in a relatively pure stand of 743 R.C. WELCH hectares. More recently, Zlatanov (1971) published an account of insect pests on 7 species of oak in Bulgaria, including Q. rubra (Table 35), although Since the early 1970s, and following a number of his lists include many pests not known to occur earlier plantings, it seems that American red oak in Britain. -

(Geometridae) in Oak Forests from Romania
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Annals of the University of Craiova - Agriculture, Montanology, Cadastre Series Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies) Voll. XLIV 2014 EVOLUTION OF INFESTATIONS WITH LOOPERMOTH (GEOMETRIDAE) IN OAK FORESTS FROM ROMANIA AUTHORS: Neţoiu Constantin, Univ. Craiova Tomescu Romică, ICAS Bucureşti Vladescu Dumitru, RNP - Romsilva Aldea Dan Ioan, RNP - Romsilva Buzatu Andrei, ICAS Craiova Keywords: Geometridae, Operophtera brumata, outbreaks, infestation. ABSTRACT In the oak forests from Romania, the species of Geometridae develops regular outbreaks with decennal frequencies. This paper presents a historic of infestations on last 52 years (1960-2012) and analyzes the evolution trend of loopermoth populations in the last outbreak in Romania (2007-2010), depending by site conditions and forest stand characteristics. The mixed forests with common oak (Quercus robur) and sessile oak (Quercus petrea) as dominant species, placed on elevated plain, or on upper sides of the southern slopes, with ages over 80 years, shown favorable for developing ample outbreaks, with exponential growth rate of loopermoth populations. 1. INTRODUCTION In deciduous forests of Romania, according with statistics from last six decades, the defoliating insects (Lepidoptera) have had the highest percentage, in terms of infested areas. From the group of defoliating insects, the infestations produced by Tortrix viridana L. represents 44%, Geometridae sp. 31,7%, Lymntria dispar L. 21.6% and other species 2,7% (Malacosoma neustria L., Euproctis chrysorrhoea L, Thaumaetopoea processionea L. etc). Between the species of Geometridae, the most frequent, in orders of its importance, are Operophtera brumata L, Erannis defoliaria Cl., Erannis aurantiaria Hb., Erannis leucophaearia Schiff., Erannis marginaria F., Phigalia pedaria F., Alsophila aescularia Schiff. -

Lepidoptera, Noctuidae, Hadeninae) Species of Iran
Turk J Zool 2012; 36(6): 752-758 © TÜBİTAK Research Article doi:10.3906/zoo-1111-15 A survey of the Perigrapha Lederer (Lepidoptera, Noctuidae, Hadeninae) species of Iran Asghar SHIRVANI1,*, Mohammad Ali SHOGHALI2, Shamsi FEIZPOOR3 1Department of Plant Protection, Faculty of Agriculture, Shahid Bahonar University of Kerman, 76169-133 Kerman – IRAN 2No. 51, 24 Azar Street, Kerman – IRAN 3Young Researchers Society, Shahid Bahonar University of Kerman, Kerman – IRAN Received: 14.11.2011 ● Accepted: 25.03.2012 Abstract: Four species of the genus Perigrapha Lederer are reviewed in Iran. Two species, P. annau Varga & Ronkay, 1991 and P. fl ora Hreblay, 1996, are reported for the fi rst time from the fauna of Iran. Adult and genitalia images are illustrated and identifi cation keys for the external and genital features are given. Key words: Perigrapha, Iran, new records, identifi cation key Introduction large-scale variation in morphological features and Th e tribe Orthosiini Guenée, 1837, with 7 genera relegated them as members of 3 genera, Anorthoa, (Panolis Hübner, [1821], Dioszeghyana Hreblay, Harutaeographa, and Perigrapha. 1993, Orthosia Ochsenheimer, 1816, Anorthoa Berio, Perigrapha, a Holarctic genus belonging to the 1980, Harutaeographa Yoshimoto, 1993, Perigrapha perigraphoid generic complex with hairy eyes typical Lederer, 1857, and Egira Duponchel, 1845), is for the subfamily Hadeninae (sensu Hampson), represented by early-fl ying, univoltine species that comprises 3 subgenera, Opacographa Hreblay, 1996, prefer mountainous and semimountainous regions Rororthosia Beck, 1999, and Perigrapha Lederer, in Iran. Th e classifi cation and taxonomic rank of 1857. Th is genus is represented in Europe by the species groups within this tribe has been a matter last 2 subgenera and 4 species (Ronkay et al., 2001). -

Moths of Poole Harbour Species List
Moths of Poole Harbour is a project of Birds of Poole Harbour Moths of Poole Harbour Species List Birds of Poole Harbour & Moths of Poole Harbour recording area The Moths of Poole Harbour Project The ‘Moths of Poole Harbour’ project (MoPH) was established in 2017 to gain knowledge of moth species occurring in Poole Harbour, Dorset, their distribution, abundance and to some extent, their habitat requirements. The study area uses the same boundaries as the Birds of Poole Harbour (BoPH) project. Abigail Gibbs and Chris Thain, previous Wardens on Brownsea Island for Dorset Wildlife Trust (DWT), were invited by BoPH to undertake a study of moths in the Poole Harbour recording area. This is an area of some 175 square kilometres stretching from Corfe Castle in the south to Canford Heath in the north of the conurbation and west as far as Wareham. 4 moth traps were purchased for the project; 3 Mercury Vapour (MV) Robinson traps with 50m extension cables and one Actinic, Ultra-violet (UV) portable Heath trap running from a rechargeable battery. This was the capability that was deployed on most of the ensuing 327 nights of trapping. Locations were selected using a number of criteria: Habitat, accessibility, existing knowledge (previously well-recorded sites were generally not included), potential for repeat visits, site security and potential for public engagement. Field work commenced from late July 2017 and continued until October. Generally, in the years 2018 – 2020 trapping field work began in March/ April and ran on until late October or early November, stopping at the first frost. -
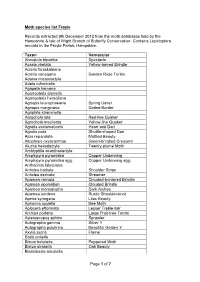
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

DE TTK 1949 Taxonomy and Systematics of the Eurasian
DE TTK 1949 Taxonomy and systematics of the Eurasian Craniophora Snellen, 1867 species (Lepidoptera, Noctuidae, Acronictinae) Az eurázsiai Craniophora Snellen, 1867 fajok taxonómiája és szisztematikája (Lepidoptera, Noctuidae, Acronictinae) PhD thesis Egyetemi doktori (PhD) értekezés Kiss Ádám Témavezető: Prof. Dr. Varga Zoltán DEBRECENI EGYETEM Természettudományi Doktori Tanács Juhász-Nagy Pál Doktori Iskola Debrecen, 2017. Ezen értekezést a Debreceni Egyetem Természettudományi Doktori Tanács Juhász-Nagy Pál Doktori Iskola Biodiverzitás programja keretében készítettem a Debreceni Egyetem természettudományi doktori (PhD) fokozatának elnyerése céljából. Debrecen, 2017. ………………………… Kiss Ádám Tanúsítom, hogy Kiss Ádám doktorjelölt 2011 – 2014. között a fent megnevezett Doktori Iskola Biodiverzitás programjának keretében irányításommal végezte munkáját. Az értekezésben foglalt eredményekhez a jelölt önálló alkotó tevékenységével meghatározóan hozzájárult. Az értekezés elfogadását javasolom. Debrecen, 2017. ………………………… Prof. Dr. Varga Zoltán A doktori értekezés betétlapja Taxonomy and systematics of the Eurasian Craniophora Snellen, 1867 species (Lepidoptera, Noctuidae, Acronictinae) Értekezés a doktori (Ph.D.) fokozat megszerzése érdekében a biológiai tudományágban Írta: Kiss Ádám okleveles biológus Készült a Debreceni Egyetem Juhász-Nagy Pál doktori iskolája (Biodiverzitás programja) keretében Témavezető: Prof. Dr. Varga Zoltán A doktori szigorlati bizottság: elnök: Prof. Dr. Dévai György tagok: Prof. Dr. Bakonyi Gábor Dr. Rácz István András -

Micro Moths on Great Cumbrae Island (Vc100)
The Glasgow Naturalist (online 2017) Volume 26, xx-xx Micro moths on Great Cumbrae Island (vc100) P. G. Moore 32 Marine Parade, Millport, Isle of Cumbrae KA28 0EF E-mail: [email protected] ABSTRACT Forsythia sp. Behind the office is a large mature Few previous records exist for miCro-moths from black mulberry tree (Morus nigra) and to one side is vC100. Data are presented from the first year-round a tall privet hedge (Ligustrum ovalifolium). To the moth-trapping exerCise accomplished on Great rear of my property is a wooded escarpment with Cumbrae Island; one of the least studied of the old-growth ash (Fraxinus excelsior) frequently ivy- Clyde Isles (vC100). Data from a Skinner-type light- Covered (Hedera helix), sycamore (Acer trap, supplemented by Collection of leaf mines from pseudoplatanus) and rowan (Sorbus aucuparia), local trees, revealed the presence of 71 species of with an undergrowth of hawthorn (Crataegus miCro moths, representing 20 new records for the monogyna), wild garliC (Allium ursinum), nettle vice-County. (Urtica dioica), bracken (Pteridium aquilinum) and bramble (Rubus fructicosus). Rhind (1988) detailed INTRODUCTION the vasCular plants found on Great Cumbrae Island The extensive nineteenth-century list of between 1985 and 1987 and delineated the history Lepidoptera in the 1901 handbook on the natural of the island's botanical investigations. Leaves of history of Glasgow and the West of SCotland issued brambles in my garden, beech trees (Fagus for the Glasgow meeting of the British AssoCiation sylvatica) and hazel (Corylus avellana) at other for the Advancement of SCience (Elliot et al., 1901) locations on the island (respectively Craiglea Wood inCluded few Cumbrae records. -

Impact of Alien Insect Pests on Sardinian Landscape and Culture
Biodiversity Journal , 2012, 3 (4): 297-310 Impact of alien insect pests on Sardinian landscape and culture Roberto A. Pantaleoni 1, 2,* , Carlo Cesaroni 1, C. Simone Cossu 1, Salvatore Deliperi 2, Leonarda Fadda 1, Xenia Fois 1, Andrea Lentini 2, Achille Loi 2, Laura Loru 1, Alessandro Molinu 1, M. Tiziana Nuvoli 2, Wilson Ramassini 2, Antonio Sassu 1, Giuseppe Serra 1, Marcello Verdinelli 1 1Istituto per lo Studio degli Ecosistemi, Consiglio Nazionale delle Ricerche (ISE-CNR), traversa la Crucca 3, Regione Baldinca, 07100 Li Punti SS, Italy; e-mail: [email protected] 2Sezione di Patologia Vegetale ed Entomologia, Dipartimento di Agraria, Università degli Studi di Sassari, via Enrico De Nicola, 07100 Sassari SS, Italy; e-mail: [email protected] *Corresponding author ABSTRACT Geologically Sardinia is a raft which, for just under thirty million years, has been crossing the western Mediterranean, swaying like a pendulum from the Iberian to the Italian Peninsula. An island so large and distant from the other lands, except for its “sister” Corsica, has inevitably developed an autochthonous flora and fauna over such a long period of time. Organisms from other Mediterranean regions have added to this original contingent. These new arrivals were not randomly distributed over time but grouped into at least three great waves. The oldest two correspond with the Messinian salinity crisis about 7 million years ago and with the ice age, when, in both periods, Sardinia was linked to or near other lands due to a fall in sea level. The third, still in progress, is linked to human activity.