PI Changes for Fibrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Statins and Lipid Lowering Agents
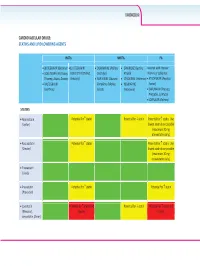
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

Joint Assessment Report Was Discussed by the Phvwp at Its Meeting in July 2007 and Finalised in September 2007
ASSESSMENT REPORT on the benefit:risk of fibrates EXECUTIVE SUMMARY 1. BACKGROUND In the light of the established role of statins in the primary and secondary prevention of cardiovascular disease (CVD) and safety concerns arising from the use of fibrates, the CHMP Pharmacovigilance Working Party (PhVWP) agreed to undertake a benefit:risk assessment of this class of medicines. The objective was to establish the current place of fibrates in the treatment of cardiovascular and dyslipidaemic diseases, and in diabetes mellitus; also to provide recommendations regarding amendments of the Summary of Product Characteristics (SPC), as necessary. Fibrates exert their effects mainly by activating the peroxisome proliferator-activated receptor-alpha (PPAR-alpha). Unique in this class, bezafibrate is an agonist for all three PPAR isoforms alpha, gamma, and delta. Fibrates have been shown to reduce plasma triglycerides by 30% to 50% and raise the level of high density lipoprotein cholesterol (HDL- C) by 2% to 20%. Their effect on low density lipoprotein cholesterol (LDL-C) is variable, ranging from no effect to a small decrease of the order of 10%. Today there are four licensed fibrates: bezafibrate, fenofibrate, gemfibrozil and ciprofibrate. Their currently approved indications are quite broad and in many cases still use the old Fredrickson classification for dyslipidaemias. 2. METHODOLOGY In February 2006 a List of Questions was agreed by the PhVWP for the Marketing Authorisation Holders (MAHs) of medicinal products containing one of the four currently licensed fibrates (Annex 1). Other clofibrate-containing medicinal products (e.g. etofibrate, etofyllinclofibrate) were excluded from this class review, since these are available only in a few member states via national marketing authorizations. -
Bezalip, 200 Mg Film Coated Tablets Bezalip Retard, 400 Mg Sustained Release, Film Coated Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Bezalip, 200 mg film coated tablets Bezalip Retard, 400 mg sustained release, film coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 200 mg of bezafibrate. Each sustained release, film coated tablets contains 400 mg of bezafibrate. Excipient with known effect: lactose monohydrate (Bezalip Retard) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Bezalip is a white, round film-coated tablet imprinted with ‘G6’ at reverse. Bezalip Retard is a white, round film-coated tablet engraved D9 on one face. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Bezalip and Bezalip is indicated for: • primary hyperlipidaemia types IIa, IIb, III, IV and V (Fredrickson classification) corresponding to groups I, II and III of the European Atherosclerosis Society guidelines – when diet alone or improvements in lifestyle such as increased exercise or weight reduction do not lead to an adequate response. • secondary hyperlipidaemias, e.g. severe hypertriglyceridemias, when sufficient improvement does not occur after correction of the underlying disorder (e.g. diabetes mellitus). 4.2 Dose and method of administration Adults The standard dosage for Bezalip 200 mg tablets is 1 tablet (200 mg) 3 times daily. In cases of good therapeutic response, especially in hypertriglyceridaemia, the dosage can be reduced to 1 tablet twice daily. For patients with a history of gastric sensitivity, the dosage may be gradually increased to the maintenance level. The standard dosage for Bezalip Retard 400 mg tablets is 1 tablet once daily. Special populations Patient with renal Impairment The dosage in patients with impaired renal function must be adjusted according to serum creatinine levels or creatinine clearance. -

(CPG) for the Management of Dyslipidemia for Cardiovascular
VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF DYSLIPIDEMIA FOR CARDIOVASCULAR RISK REDUCTION Department of Veterans Affairs Department of Defense QUALIFYING STATEMENTS The Department of Veterans Affairs and the Department of Defense guidelines are based upon the best information available at the time of publication. They are designed to provide information and assist decision making. They are not intended to define a standard of care and should not be construed as one. Neither should they be interpreted as prescribing an exclusive course of management. This Clinical Practice Guideline is based on a systematic review of both clinical trial and epidemiological evidence. Developed by a panel of multidisciplinary experts, it provides a clear explanation of the logical relationships between various care options and health outcomes while rating both the quality of the evidence and the strength of the recommendation. Variations in practice will inevitably and appropriately occur when clinicians take into account the needs of individual patients, available resources, and limitations unique to an institution or type of practice. Every healthcare professional making use of these guidelines is responsible for evaluating the appropriateness of applying them in the setting of any particular clinical situation. These guidelines are not intended to represent Department of Veterans Affairs nor TRICARE policy. Further, inclusion of recommendations for specific testing and/or therapeutic interventions within these guidelines does not guarantee coverage. Additional information on current TRICARE benefits may be found at www.tricare.mil or by contacting your regional TRICARE Managed Care Support Contractor. Version 4.0 – 2020 VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction Prepared by: The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group With support from: The Office of Quality and Patient Safety, VA, Washington, DC & Office of Evidence Based Practice, U.S. -

Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value
Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value Draft Evidence Report November 12, 2020 Prepared for ©Institute for Clinical and Economic Review, 2020 ICER Staff and Consultants Modeling Team Grace A. Lin, MD, MAS Dhruv S. Kazi, MD, MSc, MS Associate Professor of Medicine and Health Policy Associate Director, Smith Center for Outcomes University of California, San Francisco Research in Cardiology Director, Cardiac Critical Care Unit Jane Jih, MD, MPH Beth Israel Deaconess Medical Center Assistant Professor, Division of General Internal Associate Professor, Harvard Medical School Medicine University of California, San Francisco Foluso Agboola, MBBS, MPH Director, Evidence Synthesis Dr. Kazi was responsible for the development of the ICER cost-effectiveness model, interpretation of results, and drafting of the economic sections of this report; the Rick Chapman, PhD, MS resulting ICER reports do not necessarily represent the Director of Health Economics views of Beth Israel Deaconess Medical Center or ICER Harvard Medical School. Steven D. Pearson, MD, MSc President ICER DATE OF PUBLICATION: November 12, 2020 How to cite this document: Lin GA, Kazi DS, Jih J, Agboola F, Chapman R, Pearson SD. Inclisiran and Bempedoic Acid for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review, November 12, 2020. https://icer-review.org/material/high-cholesterol-update- draft-evidence-report/ Grace Lin served as the lead author for the report and wrote the background, other benefits, and contextual considerations sections of the report, with Jane Jih serving as a co-author. -

(12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee Et Al
US00779531 OB2 (12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee et al. (45) Date of Patent: Sep. 14, 2010 (54) METHODS AND REAGENTS FOR THE WO WO 2005/025673 3, 2005 TREATMENT OF METABOLIC DISORDERS OTHER PUBLICATIONS (75) Inventors: Margaret S. Lee, Middleton, MA (US); Tenenbaum et al., “Peroxisome Proliferator-Activated Receptor Grant R. Zimmermann, Somerville, Ligand Bezafibrate for Prevention of Type 2 Diabetes Mellitus in MA (US); Alyce L. Finelli, Patients With Coronary Artery Disease'. Circulation, 2004, pp. 2197 Framingham, MA (US); Daniel Grau, 22O2.* Shen et al., “Effect of gemfibrozil treatment in sulfonylurea-treated Cambridge, MA (US); Curtis Keith, patients with noninsulin-dependent diabetes mellitus'. The Journal Boston, MA (US); M. James Nichols, of Clinical Endocrinology & Metabolism, vol. 73, pp. 503-510, Boston, MA (US) 1991 (see enclosed abstract).* International Search Report from PCT/US2005/023030, mailed Dec. (73) Assignee: CombinatoRx, Inc., Cambridge, MA 1, 2005. (US) Lin et al., “Effect of Experimental Diabetes on Elimination Kinetics of Diflunisal in Rats.” Drug Metab. Dispos. 17:147-152 (1989). (*) Notice: Subject to any disclaimer, the term of this Abstract only. patent is extended or adjusted under 35 Neogi et al., “Synthesis and Structure-Activity Relationship Studies U.S.C. 154(b) by 0 days. of Cinnamic Acid-Based Novel Thiazolidinedione Antihyperglycemic Agents.” Bioorg. Med. Chem. 11:4059-4067 (21) Appl. No.: 11/171,566 (2003). Vessby et al., “Effects of Bezafibrate on the Serum Lipoprotein Lipid and Apollipoprotein Composition, Lipoprotein Triglyceride Removal (22) Filed: Jun. 30, 2005 Capacity and the Fatty Acid Composition of the Plasma Lipid Esters.” Atherosclerosis 37:257-269 (1980). -

Low Doses of Fenofibrate and Bezafibrate Stimulate Renal 2-Oxoglutarate Dehydrogenase (2-Ogdh) in Protein-Restricted Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Jagiellonian Univeristy Repository Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 77 No. 2 pp. 281ñ288, 2020 ISSN 2353-5288 DOI: 10.32383/appdr/118535 Polish Pharmaceutical Society Received 12 December 2019, Received in revised form 21 February 2020, Accepted 28 February 2020. DRUG BIOCHEMISTRY LOW DOSES OF FENOFIBRATE AND BEZAFIBRATE STIMULATE RENAL 2-OXOGLUTARATE DEHYDROGENASE (2-OGDH) IN PROTEIN-RESTRICTED RATS MALGORZATA E. KNAPIK-CZAJKA*, JAGODA M. DRAG and ANNA GAWEDZKA Department of Biochemical Analytics, Faculty of Pharmacy, Jagiellonian University Medical College, 9 Medyczna St., 30-688 KrakÛw, Poland Abstract: 2-oxoglutarate dehydrogenase (2-OGDH) is the key regulatory enzyme of cell metabolism. It has been previously demonstrated that in rats subjected to protein restriction low, clinically relevant doses of fibrates up-regulate liver 2-OGDH and promote 2-oxoglutarate catabolism. The aim of the present study was to evaluate the effect of low doses of fenofibrate and bezafibrate on renal 2-OGDH complex in rats fed low-pro- tein chow. Fibrates were administered for 14 days to Wistar male rats at one daily dose of 5, 10 or 20 mg/kg b.wt./day. The 2-OGDH activity was assayed spectrophotometrically. The mRNA levels for 2-OGDH catalyt- ic subunits (E1 and E2) and PPARα were quantified by means of semi-quantitative reverse-transcription-PCR. 2-OGDH activity increased in response to administration of fenofibrate and bezafibrate (by 11, 24, 32% and 9, 12, 21%, respectively). The difference was statistically significant for the doses of 10 and 20 mg/kg b.wt of fenofibrate (p < 0.001) and the highest dose of bezafibrate (p < 0.05). -

Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: the Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol
Diabetes Volume 65, July 2016 1767 Changting Xiao,1 Satya Dash,1 Cecilia Morgantini,1 Robert A. Hegele,2 and Gary F. Lewis1 Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol Diabetes 2016;65:1767–1778 | DOI: 10.2337/db16-0046 Notwithstanding the effectiveness of lowering LDL cho- has been the primary goal of dyslipidemia management, lesterol, residual CVD risk remains in high-risk popula- with statins as the treatment of choice for CVD prevention. tions, including patients with diabetes, likely contributed Large-scale, randomized, clinical trials of LDL-lowering PERSPECTIVES IN DIABETES to by non-LDL lipid abnormalities. In this Perspectives therapies have demonstrated significant reduction in CVD in Diabetes article, we emphasize that changing demo- events over a wide range of baseline LDL-C levels (2,3). graphics and lifestyles over the past few decades have However, even with LDL-C levels lowered substantially or “ resulted in an epidemic of the atherogenic dyslipidemia at treatment goals with statin therapy, CVD risks are not ” complex, the main features of which include hypertrigly- eliminated and there remains significant “residual risk.” In- ceridemia, low HDL cholesterol levels, qualitative changes tensifying statin therapy may provide additional benefits in LDL particles, accumulation of remnant lipoproteins, (4,5); this approach, however, has limited potential, owing and postprandial hyperlipidemia. We brieflyreviewthe to tolerability, side effects, and finite efficacy. Further LDL-C underlying pathophysiology of this form of dyslipidemia, lowering may also be achieved with the use of nonstatin in particular its association with insulin resistance, obe- sity, and type 2 diabetes, and the marked atherogenicity agents, such as cholesterol absorption inhibitors and PCSK9 of this condition. -

Fenofibrate, Bezafibrate, Ciprofibrate and Gemfibrozil Procedure Number
28 February 2011 EMA/CHMP/580013/2012 Assessment report for fenofibrate, bezafibrate, ciprofibrate, and gemfibrozil containing medicinal products pursuant to Article 31 of Directive 2001/83/EC, as amended INN: fenofibrate, bezafibrate, ciprofibrate and gemfibrozil Procedure number: EMEA/H/A-1238 Assessment Report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7051 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 3 1.1. Referral of the matter to the CHMP ......................................................................... 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction......................................................................................................... 3 2.2. Clinical aspects .................................................................................................... 4 2.2.1. PhVWP recommendation ..................................................................................... 4 2.2.2. CHMP review ..................................................................................................... 7 2.2.3. Discussion ..................................................................................................... -

Lipid Lowering Drugs Prescription and the Risk of Peripheral Neuropathy
1047 J Epidemiol Community Health: first published as 10.1136/jech.2003.013409 on 16 November 2004. Downloaded from RESEARCH REPORT Lipid lowering drugs prescription and the risk of peripheral neuropathy: an exploratory case-control study using automated databases Giovanni Corrao, Antonella Zambon, Lorenza Bertu`, Edoardo Botteri, Olivia Leoni, Paolo Contiero ............................................................................................................................... J Epidemiol Community Health 2004;58:1047–1051. doi: 10.1136/jech.2003.013409 Study objective: Although lipid lowering drugs are effective in preventing morbidity and mortality from cardiovascular events, the extent of their adverse effects is not clear. This study explored the association between prescription of lipid lowering drugs and the risk of peripheral neuropathy. Design: A population based case-control study was carried out by linkage of several automated databases. Setting: Resident population of a northern Italian Province aged 40 years or more. Participants: Cases were patients discharged for peripheral neuropathy in 1998–1999. For each case up See end of article for authors’ affiliations to 20 controls were randomly selected among those eligible. Altogether 2040 case patients and 36 041 ....................... controls were included in the study. Exposure ascertainment: Prescription drug database was used to assess exposure to lipid lowering drugs Correspondence to: Professor G Corrao, at any time in the one year period preceding the index date.