Treatment Strategy for Dyslipidemia in Cardiovascular Disease Prevention: Focus on Old and New Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

125559Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125559Orig1s000 PHARMACOLOGY REVIEW(S) Tertiary Pharmacology/Toxicology Review Date: July 14, 2015 From: Timothy J. McGovern, Ph.D., ODE Associate Director for Pharmacology and Toxicology, OND IO BLA: 125559 Agency receipt date: November 24, 2014 Drug: PRALUENT (alirocumab) Sponsor: Sanofi-Aventis U.S. LLC Indication: Adult patients with primary hypercholesterolemia (non-familial and heterozygous familial) or mixed dyslipidemia Reviewing Division: Division of Metabolism and Endocrinology Products Introductory Comments: The pharmacology/toxicology reviewer and supervisor concluded that the nonclinical data support approval of PRALUENT (alirocumab) for the indication listed above. Alirocumab is a human IgG1 monoclonal antibody that binds to human PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9). The recommended Established Pharmacologic Class for alirocumab is PCSK9 inhibitor antibody. There are no approved products in this class currently. An appropriate nonclinical program was conducted by the sponsor to support approval of alirocumab. Alirocumab elicited expected pharmacological responses in rats, hamsters, and monkeys; alirocumab lowered total cholesterol and LDL-cholesterol in the species tested and decreased HDL-cholesterol in rats and hamsters. The primary nonclinical toxicity studies of alirocumab were conducted in rats and monkeys for up to 6 months duration with weekly subcutaneous and intravenous dosing. No significant adverse findings were observed at the doses tested which achieved exposure multiples up to 11-fold in rats and 103-fold in monkeys compared to the maximum recommended human dose of 150 mg alirocumab administered subcutaneously once every two weeks. Findings in the liver and adrenal glands of rats were associated with exaggerated pharmacologic effects. -

Ezetimibe: a Novel Selective Cholesterol Absorption Inhibitor by Michele Koder, Pharm.D
OREGON DUR BOARD NEWSLETTER A N E VIDENCE B ASED D RUG T HERAPY R ESOURCE COPYRIGHT 2003 OREGON STATE UNIVERSITY. ALL RIGHTS RESERVED Volume 5, Issue 2 Also available on the web and via e-mail list-serve at February 2003 http://pharmacy.orst.edu/drug_policy/newsletter_email.html Ezetimibe: A novel selective cholesterol absorption inhibitor By Michele Koder, Pharm.D. , OSU College of Pharmacy Ezetimibe (Zetia) is a novel selective cholesterol absorption inhibitor that was approved by the FDA in October 2002. Unlike statins (HMG-CoA reductase inhibitors) and bile acid sequestrants, ezetimibe does not inhibit hepatic cholesterol synthesis or increase bile acid secretion. In contrast, ezetimibe selectively inhibits the uptake of dietary cholesterol from enterocytes in the brush border of the intestinal lumen resulting in a decrease in the delivery of dietary cholesterol to the liver and a subsequent decrease in hepatic cholesterol stores and increased cholesterol clearance from the blood.1 Ezetimibe’s unique action has generated interest in its use in combination with other cholesterol-lowering agents. It is indicated for the treatment of primary hypercholesterolemia as monotherapy and in combination with a statin. Ezetimibe is also approved for homozygous familial hypercholesterolemia and homozygous sitosterolemia. TABLE 1: EZETIMIBE CLINICAL TRIAL SUMMARY Study / Design Population Treatment % Change LDL % Change HDL % Change TG Bays et al3 N=432 EZ 5 mg -15.7 +2.9 MC, R, DB, PC LDL 130-250mg/dl EZ 10 mg -18.5 +3.5 NS 12 wk; Phase II TG -

Nustendi, INN-Bempedoic Acid, Ezetimibe
Summary of risk management plan for Nustendi (Bempedoic acid/Ezetimibe) This is a summary of the risk management plan (RMP) for Nustendi. The RMP details important risks of Nustendi, how these risks can be minimized, and how more information will be obtained about Nustendi's risks and uncertainties (missing information). Nustendi's summary of product characteristics (SmPC) and its package leaflet give essential information to healthcare professionals and patients on how Nustendi should be used. This summary of the RMP for Nustendi should be read in the context of all this information, including the assessment report of the evaluation and its plain-language summary, all which is part of the European Public Assessment Report (EPAR). Important new concerns or changes to the current ones will be included in updates of Nustendi's RMP. I. The Medicine and What It Is Used For Nustendi is authorized for treatment of primary hypercholesterolemia in adults, as an adjunct to diet (see SmPC for the full indication). It contains bempedoic acid as the active substance and it is given by mouth. Further information about the evaluation of Nustendi’s benefits can be found in Nustendi’s EPAR, including in its plain-language summary, available on the EMA website, under the medicine’s webpage https://www.ema.europa.eu/en/medicines/human/EPAR/nustendi II. Risks Associated With the Medicine and Activities to Minimize or Further Characterize the Risks Important risks of Nustendi, together with measures to minimize such risks and the proposed studies for learning -

Pharmacy Prior Authorization Non-Formulary and Prior
Pharmacy Prior Authorization Non-Formulary and Prior Authorization Guidelines Scroll down to see PA Criteria by drug class, or Ctrl+F to search document by drug name PA Guideline Requirements Duration of Approval if Requirements Are Met Non-Formulary Requests for Non-Formulary Medications that do not have specific Prior Authorization Initial Approval: Medication Guidelines will be reviewed based on the following: • Minimum of 3 months, Guideline • An appropriate diagnosis/indication for the requested medication, depending on the • An appropriate dose of medication based on age and indication, diagnosis, to determine • Documented trial of 2 formulary agents for an adequate duration have not been effective or adherence, efficacy and tolerated patient safety monitoring OR • All other formulary medications are contraindicated based on the patient’s diagnosis, other Renewal: medical conditions or other medication therapy, • Minimum of 6 months OR • Maintenance medications • There are no other medications available on the formulary to treat the patient’s condition may be approved Indefinite Maryland Physicians Care determines patient medication trials and adherence by a review of pharmacy claims data over the preceding twelve months. Additional information may be requested on a case-by-case basis to allow for proper review. Medications Requests for Medications requiring Prior Authorization (PA) will be reviewed based on the PA As documented in the requiring Prior Guidelines/Criteria for that medication. Scroll down to view the PA Guidelines for specific individual guideline Authorization medications. Medications that do not have a specific PA guideline will follow the Non-Formulary Medication Guideline. Additional information may be required on a case-by-case basis to allow for adequate review. -

Lipid-Lowering Therapy and Low-Density Lipoprotein Cholesterol
Kristensen et al. BMC Cardiovascular Disorders (2020) 20:336 https://doi.org/10.1186/s12872-020-01616-9 RESEARCH ARTICLE Open Access Lipid-lowering therapy and low-density lipoprotein cholesterol goal attainment after acute coronary syndrome: a Danish population-based cohort study Marie Skov Kristensen1, Anders Green2,3, Mads Nybo4, Simone Møller Hede2, Kristian Handberg Mikkelsen5, Gunnar Gislason1,6,7,8, Mogens Lytken Larsen9 and Annette Kjær Ersbøll1* Abstract Background: Patients with acute coronary syndrome (ACS) are at high risk of recurrent cardiovascular (CV) event. The European guidelines recommend low-density lipoprotein cholesterol (LDL-C) levels < 1.8 mmol/L and early initiation of intensive lipid-lowering therapy (LLT) to reduce CV risk. In order to reduce the risk of further cardiac events, the study aimed to evaluate LDL-C goal attainment and LLT intensity in an incident ACS population. Methods: A cohort study of patients with residency at Funen in Denmark at a first-ever ACS event registered within the period 2010–2015. Information on LLT use and LDL-C levels was extracted from national population registers and a Laboratory database at Odense University Hospital. Treatments and lipid patterns were evaluated during index hospitalization, at 6-month and 12-month follow-up. Results: Among 3040 patients with an LDL-C measurement during index hospitalization, 40.7 and 39.0% attained the recommended LDL-C target value (< 1.8 mmol/L) within 6- and 12-month follow-up, respectively. During 6- and 12-month follow-up, a total of 89.2% (20.2%) and 88.4% (29.7%) used LLT (intensive LLT). -

Repatha) Reference Number: PA.CP.PHAR.123 Effective Date: 01/18 Last Review Date: 01/19 Revision Log
Clinical Policy: Evolocumab (Repatha) Reference Number: PA.CP.PHAR.123 Effective Date: 01/18 Last Review Date: 01/19 Revision Log Description Evolocumab (RepathaTM) is a proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitor antibody. FDA Approved Indication(s) Repatha is indicated: • To reduce the risk of myocardial infarction, stroke, and coronary revascularization in adults with established cardiovascular disease • As an adjunct to diet, alone, or in combination with other lipid-lowering therapies (e.g., statins, ezetimibe), for treatment of adults with primary hyperlipidemia (including heterozygous familial hypercholesterolemia) to reduce low-density lipoprotein cholesterol (LDL-C) • As an adjunct to diet and other LDL-lowering therapies (e.g., statins, ezetimibe, LDL apheresis) in patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C Policy/Criteria Provider must submit documentation (which may include office chart notes and lab results) supporting that member has met all approval criteria It is the policy of health plans affiliated with Pennsylvania Health and Wellness that Repatha is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Heterozygous Familial Hypercholesterolemia and Atherosclerotic Cardiovascular Disease (must meet all): 1. Diagnosis of one of the following (a or b): a. HeFH as defined by one of the following (i or ii): i. World Health Organization (WHO)/Dutch Lipid Network familial hypercholesterolemia diagnostic criteria score of > 8 as determined by requesting provider; ii. Definite diagnosis per Simon Broome criteria); b. Atherosclerotic cardiovascular disease (ASCVD) as evidenced by a history of any one of the following conditions (i-vii): i. Acute coronary syndromes; ii. -

Medicines That Affect Fluid Balance in the Body
the bulk of stools by getting them to retain liquid, which encourages the Medicines that affect fluid bowels to push them out. balance in the body Osmotic laxatives e.g. Lactulose, Macrogol - these soften stools by increasing the amount of water released into the bowels, making them easier to pass. Older people are at higher risk of dehydration due to body changes in the ageing process. The risk of dehydration can be increased further when Stimulant laxatives e.g. Senna, Bisacodyl - these stimulate the bowels elderly patients are prescribed medicines for chronic conditions due to old speeding up bowel movements and so less water is absorbed from the age. stool as it passes through the bowels. Some medicines can affect fluid balance in the body and this may result in more water being lost through the kidneys as urine. Stool softener laxatives e.g. Docusate - These can cause more water to The medicines that can increase risk of dehydration are be reabsorbed from the bowel, making the stools softer. listed below. ANTACIDS Antacids are also known to cause dehydration because of the moisture DIURETICS they require when being absorbed by your body. Drinking plenty of water Diuretics are sometimes called 'water tablets' because they can cause you can reduce the dry mouth, stomach cramps and dry skin that is sometimes to pass more urine than usual. They work on the kidneys by increasing the associated with antacids. amount of salt and water that comes out through the urine. Diuretics are often prescribed for heart failure patients and sometimes for patients with The major side effect of antacids containing magnesium is diarrhoea and high blood pressure. -
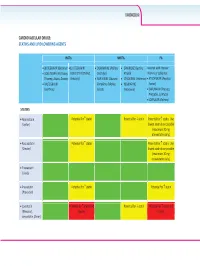
Statins and Lipid Lowering Agents
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy by Anthony C
Supplement to Supported by an unrestricted educational grant from Abbott Laboratories March/April 2008 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy By Anthony C. Keech, MBBS, Msc Epid, FRANZCS, FRACP; and Paul Mitchell, MBBS(Hons), MD, PhD, FRANZCO, FRACS, FRCOphth, FAFPHM This agent’s mechanism of benefit in diabetic retinopathy appears to go beyond its effects on lipid concentration or blood pressure, and this potential mechanism of action operates even when glycemic control and blood pressure levels are within goal. ABSTRACT icant relative reduction was seen of almost one-third in PURPOSE the rate of first laser application for retinopathy after The FIELD (Fenofibrate Intervention and Event an average treatment duration of 5 years with fenofi- Lowering in Diabetes) study sought to investigate brate 200 mg/day. whether long-term lipid-lowering therapy with fenofi- In this report, we detail the effects of fenofibrate brate would reduce macro- and microvascular compli- administration on ophthalmic microvascular compli- cations among patients with type 2 diabetes. We previ- cations and attempt to clarify some of the underlying ously reported that in type 2 diabetes patients with pathologies being addressed among patients undergo- adequate glycemic and blood pressure control, a signif- ing laser treatment. Jointly sponsored by The Dulaney Foundation and Retina Today MARCH/APRIL 2008 I SUPPLEMENT TO RETINA TODAY I 1 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy Jointly sponsored by The Dulaney Foundation and Retina Today. Release date: April 2008. Expiration date: April 2009. This continuing medical education activity is supported by an unrestricted educational grant from Abbott Laboratories. -

Pharmacological Agents Currently in Clinical Trials for Disorders in Neurogastroenterology
Pharmacological agents currently in clinical trials for disorders in neurogastroenterology Michael Camilleri J Clin Invest. 2013;123(10):4111-4120. https://doi.org/10.1172/JCI70837. Clinical Review Esophageal, gastrointestinal, and colonic diseases resulting from disorders of the motor and sensory functions represent almost half the patients presenting to gastroenterologists. There have been significant advances in understanding the mechanisms of these disorders, through basic and translational research, and in targeting the receptors or mediators involved, through clinical trials involving biomarkers and patient responses. These advances have led to relief of patients’ symptoms and improved quality of life, although there are still significant unmet needs. This article reviews the pipeline of medications in development for esophageal sensorimotor disorders, gastroparesis, chronic diarrhea, chronic constipation (including opioid-induced constipation), and visceral pain. Find the latest version: https://jci.me/70837/pdf Review Pharmacological agents currently in clinical trials for disorders in neurogastroenterology Michael Camilleri Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota, USA. Esophageal, gastrointestinal, and colonic diseases resulting from disorders of the motor and sensory functions represent almost half the patients presenting to gastroenterologists. There have been significant advances in under- standing the mechanisms of these disorders, through basic and translational research, and in targeting the recep- tors or mediators involved, through clinical trials involving biomarkers and patient responses. These advances have led to relief of patients’ symptoms and improved quality of life, although there are still significant unmet needs. This article reviews the pipeline of medications in development for esophageal sensorimotor disorders, gastropa- resis, chronic diarrhea, chronic constipation (including opioid-induced constipation), and visceral pain. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

Statins: Mechanism of Action and Effects
J.Cell.Mol.Med. Vol 5, No 4, 2001 pp. 378-387 Review Statins: mechanism of action and effects Camelia Stancu, Anca Sima * "Nicolae Simionescu" Institute of Cellular Biology and Pathology, Bucharest, Romania Received: November 12, 2001; Accepted: December 5, 2001 • Introduction • Effects on • Classification of statins - cholesterol esterification and - How they are obtained its accumulation in macrophages - Liver metabolism - endothelial cell function - Physico-chemical properties - inflammatory process - Specific activity - proliferation, migration and • Mechanisms for the action of statins apoptosis of arterial smooth muscle cells - Mechanisms involving lipids - stability of the atherosclerotic plaque - Mechanisms involving intracellular - platelets activation signaling pathways - coagulation process • Beneficial effects of statins • Adverse effects of statins therapy • Conclusions Abstract The beneficial effects of statins are the result of their capacity to reduce cholesterol biosyntesis, mainly in the liver, where they are selectively distributed, as well as to the modulation of lipid metabolism, derived from their effect of inhibition upon HMG-CoA reductase. Statins have antiatherosclerotic effects, that positively correlate with the percent decrease in LDL cholesterol. In addition, they can exert antiatherosclerotic effects independently of their hypolipidemic action. Because the mevalonate metabolism generates a series of isoprenoids vital for different cellular functions, from cholesterol synthesis to the control of cell growth and differentiation, HMG-CoA reductase inhibition has beneficial pleiotropic effects. Consequently, statins reduce significantly the incidence of coronary events, both in primary and secondary prevention, being the most efficient hypolipidemic compounds that have reduced the rate of mortality in coronary patients. Independent of their hypolipidemic properties, statins interfere with events involved in bone formation and impede tumor cell growth.