(CPG) for the Management of Dyslipidemia for Cardiovascular
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -
Statins and Lipid Lowering Agents
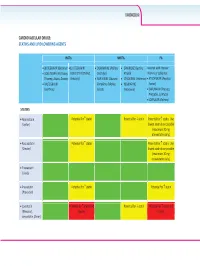
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

HMG-Coa Reductase (HMGCR)
HMG-CoA Reductase (HMGCR) HMG-CoA Reductase (HMGCR) is the rate-limiting enzyme for cholesterol synthesis in the conversion of HMG-CoA to mevalonate. HMGCR is found in eukaryotes and prokaryotes. The phylogenetic analysis has revealed two classes of HMG-CoA reductase, the Class I enzymes of eukaryotes and some archaea and the Class II enzymes of eubacteria and certain other archaea. Both in eukaryotes and in archaebacteria the enzyme HMGCR is known to catalyze an early reaction unique to isoprenoid biosynthesis. In humans, the HMG-CoA reductase reaction is rate-limiting for the biosynthesis of cholesterol and therefore constitutes a prime target of drugs that reduce serum cholesterol levels. www.MedChemExpress.com 1 HMG-CoA Reductase (HMGCR) Inhibitors (3R,5R)-Rosuvastatin (3R,5S)-Fluvastatin Cat. No.: HY-17504C ((3R,5S)-XU 62-320 free acid) Cat. No.: HY-14664B (3R,5R)-Rosuvastatin is the (3R,5R)-enantiomer of (3R,5S)-Fluvastatin is the Rosuvastatin. Rosuvastatin is a competitive 3R,5S-isomer Fluvastatin. Fluvastatin (XU HMG-CoA reductase inhibitor with an IC50 of 11 62-320 free acid) is a first fully synthetic, nM. Rosuvastatin potently blocks human competitive HMG-CoA reductase inhibitor with ether-a-go-go related gene (hERG) current with an an IC50 of 8 nM. IC50 of 195 nM. Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg, 5 mg Size: 1 mg, 5 mg (3S,5R)-Fluvastatin D6 sodium (3S,5R)-Fluvastatin sodium ((3S,5R)-XU 62-320 D6) Cat. No.: HY-14664CS ((3S,5R)-XU 62-320) Cat. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

The Effectiveness of Statins for Primary Prevention: a Rapid Review
The Effectiveness of Statins for Primary Prevention: A Rapid Review A Schaink February 2013 Effectiveness of Statins for Primary Prevention: A Rapid Review. February 2013; pp. 1–23. Suggested Citation This report should be cited as follows: Schaink A. The effectiveness of statins for primary prevention: a rapid review. Toronto, ON: Health Quality Ontario; 2013 Feb. 23 p. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac- recommendations/rapid-reviews. Conflict of Interest Statement All reports prepared by the Division of Evidence Development and Standards at Health Quality Ontario are impartial. There are no competing interests or conflicts of interest to declare. Rapid Review Methodology Clinical questions are developed by the Division of Evidence Development and Standards at Health Quality Ontario in consultation with experts, end-users, and/or applicants in the topic area. A systematic literature search is then conducted to identify relevant systematic reviews, health technology assessments, and meta-analyses; if none are located, the search is expanded to include randomized controlled trials (RCTs), and guidelines. Systematic reviews are evaluated using a rating scale developed for this purpose. If the systematic review has evaluated the included primary studies using the GRADE Working Group criteria (http://www.gradeworkinggroup.org/index.htm), the results are reported and the rapid review process is complete. If the systematic review has not evaluated the primary studies using GRADE, the primary studies included in the systematic review are retrieved and a maximum of two outcomes are graded. If no well-conducted systematic reviews are available, RCTs and/or guidelines are evaluated. Because rapid reviews are completed in very short timeframes, other publication types are not included. -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -
Bezalip, 200 Mg Film Coated Tablets Bezalip Retard, 400 Mg Sustained Release, Film Coated Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Bezalip, 200 mg film coated tablets Bezalip Retard, 400 mg sustained release, film coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 200 mg of bezafibrate. Each sustained release, film coated tablets contains 400 mg of bezafibrate. Excipient with known effect: lactose monohydrate (Bezalip Retard) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Bezalip is a white, round film-coated tablet imprinted with ‘G6’ at reverse. Bezalip Retard is a white, round film-coated tablet engraved D9 on one face. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Bezalip and Bezalip is indicated for: • primary hyperlipidaemia types IIa, IIb, III, IV and V (Fredrickson classification) corresponding to groups I, II and III of the European Atherosclerosis Society guidelines – when diet alone or improvements in lifestyle such as increased exercise or weight reduction do not lead to an adequate response. • secondary hyperlipidaemias, e.g. severe hypertriglyceridemias, when sufficient improvement does not occur after correction of the underlying disorder (e.g. diabetes mellitus). 4.2 Dose and method of administration Adults The standard dosage for Bezalip 200 mg tablets is 1 tablet (200 mg) 3 times daily. In cases of good therapeutic response, especially in hypertriglyceridaemia, the dosage can be reduced to 1 tablet twice daily. For patients with a history of gastric sensitivity, the dosage may be gradually increased to the maintenance level. The standard dosage for Bezalip Retard 400 mg tablets is 1 tablet once daily. Special populations Patient with renal Impairment The dosage in patients with impaired renal function must be adjusted according to serum creatinine levels or creatinine clearance. -

Regulation of Pharmaceutical Prices: Evidence from a Reference Price Reform in Denmark
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Kaiser, Ulrich; Mendez, Susan J.; Rønde, Thomas Working Paper Regulation of pharmaceutical prices: Evidence from a reference price reform in Denmark ZEW Discussion Papers, No. 10-062 Provided in Cooperation with: ZEW - Leibniz Centre for European Economic Research Suggested Citation: Kaiser, Ulrich; Mendez, Susan J.; Rønde, Thomas (2010) : Regulation of pharmaceutical prices: Evidence from a reference price reform in Denmark, ZEW Discussion Papers, No. 10-062, Zentrum für Europäische Wirtschaftsforschung (ZEW), Mannheim This Version is available at: http://hdl.handle.net/10419/41440 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. www.econstor.eu Dis cus si on Paper No. 10-062 Regulation of Pharmaceutical Prices: Evidence from a Reference Price Reform in Denmark Ulrich Kaiser, Susan J. -

(12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee Et Al
US00779531 OB2 (12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee et al. (45) Date of Patent: Sep. 14, 2010 (54) METHODS AND REAGENTS FOR THE WO WO 2005/025673 3, 2005 TREATMENT OF METABOLIC DISORDERS OTHER PUBLICATIONS (75) Inventors: Margaret S. Lee, Middleton, MA (US); Tenenbaum et al., “Peroxisome Proliferator-Activated Receptor Grant R. Zimmermann, Somerville, Ligand Bezafibrate for Prevention of Type 2 Diabetes Mellitus in MA (US); Alyce L. Finelli, Patients With Coronary Artery Disease'. Circulation, 2004, pp. 2197 Framingham, MA (US); Daniel Grau, 22O2.* Shen et al., “Effect of gemfibrozil treatment in sulfonylurea-treated Cambridge, MA (US); Curtis Keith, patients with noninsulin-dependent diabetes mellitus'. The Journal Boston, MA (US); M. James Nichols, of Clinical Endocrinology & Metabolism, vol. 73, pp. 503-510, Boston, MA (US) 1991 (see enclosed abstract).* International Search Report from PCT/US2005/023030, mailed Dec. (73) Assignee: CombinatoRx, Inc., Cambridge, MA 1, 2005. (US) Lin et al., “Effect of Experimental Diabetes on Elimination Kinetics of Diflunisal in Rats.” Drug Metab. Dispos. 17:147-152 (1989). (*) Notice: Subject to any disclaimer, the term of this Abstract only. patent is extended or adjusted under 35 Neogi et al., “Synthesis and Structure-Activity Relationship Studies U.S.C. 154(b) by 0 days. of Cinnamic Acid-Based Novel Thiazolidinedione Antihyperglycemic Agents.” Bioorg. Med. Chem. 11:4059-4067 (21) Appl. No.: 11/171,566 (2003). Vessby et al., “Effects of Bezafibrate on the Serum Lipoprotein Lipid and Apollipoprotein Composition, Lipoprotein Triglyceride Removal (22) Filed: Jun. 30, 2005 Capacity and the Fatty Acid Composition of the Plasma Lipid Esters.” Atherosclerosis 37:257-269 (1980). -

Low Doses of Fenofibrate and Bezafibrate Stimulate Renal 2-Oxoglutarate Dehydrogenase (2-Ogdh) in Protein-Restricted Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Jagiellonian Univeristy Repository Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 77 No. 2 pp. 281ñ288, 2020 ISSN 2353-5288 DOI: 10.32383/appdr/118535 Polish Pharmaceutical Society Received 12 December 2019, Received in revised form 21 February 2020, Accepted 28 February 2020. DRUG BIOCHEMISTRY LOW DOSES OF FENOFIBRATE AND BEZAFIBRATE STIMULATE RENAL 2-OXOGLUTARATE DEHYDROGENASE (2-OGDH) IN PROTEIN-RESTRICTED RATS MALGORZATA E. KNAPIK-CZAJKA*, JAGODA M. DRAG and ANNA GAWEDZKA Department of Biochemical Analytics, Faculty of Pharmacy, Jagiellonian University Medical College, 9 Medyczna St., 30-688 KrakÛw, Poland Abstract: 2-oxoglutarate dehydrogenase (2-OGDH) is the key regulatory enzyme of cell metabolism. It has been previously demonstrated that in rats subjected to protein restriction low, clinically relevant doses of fibrates up-regulate liver 2-OGDH and promote 2-oxoglutarate catabolism. The aim of the present study was to evaluate the effect of low doses of fenofibrate and bezafibrate on renal 2-OGDH complex in rats fed low-pro- tein chow. Fibrates were administered for 14 days to Wistar male rats at one daily dose of 5, 10 or 20 mg/kg b.wt./day. The 2-OGDH activity was assayed spectrophotometrically. The mRNA levels for 2-OGDH catalyt- ic subunits (E1 and E2) and PPARα were quantified by means of semi-quantitative reverse-transcription-PCR. 2-OGDH activity increased in response to administration of fenofibrate and bezafibrate (by 11, 24, 32% and 9, 12, 21%, respectively). The difference was statistically significant for the doses of 10 and 20 mg/kg b.wt of fenofibrate (p < 0.001) and the highest dose of bezafibrate (p < 0.05). -

Metabolic Enzyme/Protease
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com Metabolic Enzyme/Protease Metabolic pathways are enzyme-mediated biochemical reactions that lead to biosynthesis (anabolism) or breakdown (catabolism) of natural product small molecules within a cell or tissue. In each pathway, enzymes catalyze the conversion of substrates into structurally similar products. Metabolic processes typically transform small molecules, but also include macromolecular processes such as DNA repair and replication, and protein synthesis and degradation. Metabolism maintains the living state of the cells and the organism. Proteases are used throughout an organism for various metabolic processes. Proteases control a great variety of physiological processes that are critical for life, including the immune response, cell cycle, cell death, wound healing, food digestion, and protein and organelle recycling. On the basis of the type of the key amino acid in the active site of the protease and the mechanism of peptide bond cleavage, proteases can be classified into six groups: cysteine, serine, threonine, glutamic acid, aspartate proteases, as well as matrix metalloproteases. Proteases can not only activate proteins such as cytokines, or inactivate them such as numerous repair proteins during apoptosis, but also expose cryptic sites, such as occurs with β-secretase during amyloid precursor protein processing, shed various transmembrane proteins such as occurs with metalloproteases and cysteine proteases, or convert receptor agonists into antagonists and vice versa such as chemokine conversions carried out by metalloproteases, dipeptidyl peptidase IV and some cathepsins. In addition to the catalytic domains, a great number of proteases contain numerous additional domains or modules that substantially increase the complexity of their functions. -

Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: the Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol
Diabetes Volume 65, July 2016 1767 Changting Xiao,1 Satya Dash,1 Cecilia Morgantini,1 Robert A. Hegele,2 and Gary F. Lewis1 Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol Diabetes 2016;65:1767–1778 | DOI: 10.2337/db16-0046 Notwithstanding the effectiveness of lowering LDL cho- has been the primary goal of dyslipidemia management, lesterol, residual CVD risk remains in high-risk popula- with statins as the treatment of choice for CVD prevention. tions, including patients with diabetes, likely contributed Large-scale, randomized, clinical trials of LDL-lowering PERSPECTIVES IN DIABETES to by non-LDL lipid abnormalities. In this Perspectives therapies have demonstrated significant reduction in CVD in Diabetes article, we emphasize that changing demo- events over a wide range of baseline LDL-C levels (2,3). graphics and lifestyles over the past few decades have However, even with LDL-C levels lowered substantially or “ resulted in an epidemic of the atherogenic dyslipidemia at treatment goals with statin therapy, CVD risks are not ” complex, the main features of which include hypertrigly- eliminated and there remains significant “residual risk.” In- ceridemia, low HDL cholesterol levels, qualitative changes tensifying statin therapy may provide additional benefits in LDL particles, accumulation of remnant lipoproteins, (4,5); this approach, however, has limited potential, owing and postprandial hyperlipidemia. We brieflyreviewthe to tolerability, side effects, and finite efficacy. Further LDL-C underlying pathophysiology of this form of dyslipidemia, lowering may also be achieved with the use of nonstatin in particular its association with insulin resistance, obe- sity, and type 2 diabetes, and the marked atherogenicity agents, such as cholesterol absorption inhibitors and PCSK9 of this condition.