What Is Showa Denko Chloroprene?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acquisition of Printed Wiring Board Business of Showa Denko Materials Co., Ltd
Polaris Capital Group Co., Ltd. June 7, 2021 Acquisition of Printed Wiring Board Business of Showa Denko Materials Co., Ltd. and Its Subsidiaries Polaris Private Equity Fund V, L.P., etc., managed by Polaris Capital Group Co., Ltd. (“Polaris”) have recently signed an agreement with Showa Denko Materials Co., Ltd. (“Showa Denko Materials”) to acquire all the shares outstanding of a new company (the “Newly-established Company”) established to succeed Printed Wiring Board (“PWB”) business operated by Showa Denko Materials and its subsidiaries (the “Target Business”). Based on its excellence in R&D and technical capabilities developed through its more than 50 years of operations in the global PWB market, Target Business offers a wide range of products with unique strengths, including ultra-high-density Multi Wiring Boards (“MWB”) (used for probe card for high- end DRAM testing) holding a dominant market share, high precision module PWB (used for RF module for 5G smartphone, etc.), Multilayer printed wiring Boards (“MLB”) for high-speed application (used for 400Gbps switch for data center, etc.), and aluminum-based PWB (used for automotive LED lighting, etc.). Target Business has won high customer satisfaction and therefore built strong relationships with leading players in various industries, leveraging its high yield rate and low defect rate, which are essential for winning in the high-end markets. Target Business maintains the leading position in the global PWB market backed by five manufacturing sites in Japan and Singapore, each of which -
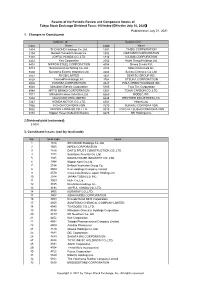
Published on July 21, 2021 1. Changes in Constituents 2
Results of the Periodic Review and Component Stocks of Tokyo Stock Exchange Dividend Focus 100 Index (Effective July 30, 2021) Published on July 21, 2021 1. Changes in Constituents Addition(18) Deletion(18) CodeName Code Name 1414SHO-BOND Holdings Co.,Ltd. 1801 TAISEI CORPORATION 2154BeNext-Yumeshin Group Co. 1802 OBAYASHI CORPORATION 3191JOYFUL HONDA CO.,LTD. 1812 KAJIMA CORPORATION 4452Kao Corporation 2502 Asahi Group Holdings,Ltd. 5401NIPPON STEEL CORPORATION 4004 Showa Denko K.K. 5713Sumitomo Metal Mining Co.,Ltd. 4183 Mitsui Chemicals,Inc. 5802Sumitomo Electric Industries,Ltd. 4204 Sekisui Chemical Co.,Ltd. 5851RYOBI LIMITED 4324 DENTSU GROUP INC. 6028TechnoPro Holdings,Inc. 4768 OTSUKA CORPORATION 6502TOSHIBA CORPORATION 4927 POLA ORBIS HOLDINGS INC. 6503Mitsubishi Electric Corporation 5105 Toyo Tire Corporation 6988NITTO DENKO CORPORATION 5301 TOKAI CARBON CO.,LTD. 7011Mitsubishi Heavy Industries,Ltd. 6269 MODEC,INC. 7202ISUZU MOTORS LIMITED 6448 BROTHER INDUSTRIES,LTD. 7267HONDA MOTOR CO.,LTD. 6501 Hitachi,Ltd. 7956PIGEON CORPORATION 7270 SUBARU CORPORATION 9062NIPPON EXPRESS CO.,LTD. 8015 TOYOTA TSUSHO CORPORATION 9101Nippon Yusen Kabushiki Kaisha 8473 SBI Holdings,Inc. 2.Dividend yield (estimated) 3.50% 3. Constituent Issues (sort by local code) No. local code name 1 1414 SHO-BOND Holdings Co.,Ltd. 2 1605 INPEX CORPORATION 3 1878 DAITO TRUST CONSTRUCTION CO.,LTD. 4 1911 Sumitomo Forestry Co.,Ltd. 5 1925 DAIWA HOUSE INDUSTRY CO.,LTD. 6 1954 Nippon Koei Co.,Ltd. 7 2154 BeNext-Yumeshin Group Co. 8 2503 Kirin Holdings Company,Limited 9 2579 Coca-Cola Bottlers Japan Holdings Inc. 10 2914 JAPAN TOBACCO INC. 11 3003 Hulic Co.,Ltd. 12 3105 Nisshinbo Holdings Inc. 13 3191 JOYFUL HONDA CO.,LTD. -

Highlights of Major General Chemical Manufacturers' Financial Results for Fiscal Year Ended March 2021
21-D-0223 June 7, 2021 Highlights of Major General Chemical Manufacturers’ Financial Results for Fiscal Year Ended March 2021 The following are Japan Credit Rating Agency, Ltd. (JCR)’s perception of the current status and highlights for rating concerning the financial results for the fiscal year ended March 2021 (FY2020) and earnings forecasts for FY2021 of Japan’s 7 general chemical manufacturers: ASAHI KASEI CORPORATION, Showa Denko K.K. (with January-December accounting period), SUMITOMO CHEMICAL COMPANY, LIMITED, TOSOH CORPORATION, Mitsui Chemicals, Inc., Mitsubishi Chemical Holdings Corporation (“Mitsubishi Chemical HD”) and Ube Industries, Ltd. 1. Industry Trend In recent years, business environment of chemical industry has been somewhat severe. Since 2018, trade friction between the U.S. and China has been a negative factor, and in 2020, the COVID-19 pandemic broke out. The COVID-19 pandemic has had a significant negative impact on social and economic activities around the world. Although uncertainty about the future of economic trend has lessened compared to the time when the pandemic broke out, the containment cannot still be foreseen. With regard to bulk chemicals, average utilization rate of domestic ethylene centers in fiscal 2020 was 93.9% (compared to an average of 94.2% in fiscal 2019), falling below the full utilization level of 95% for the second consecutive year. However, the monthly capacity utilization rate hovered around 90% in the first half of the fiscal year, but began to pick up in the second half, with some months exceeding 95%. In addition to recoveries in consumer activity and demand for downstream products, recent cold wave in the U.S. -

Comparative Durability and Abrasion Resistance of Natural and Synthetic
Comparative Durability and Abrasion Resistance of Natural and Synthetic Latex Gloves Ryan Michel2 and Katrina Cornish1,2 Department of Horticulture and Crop Science, 2Department of Food, Agricultural and Biological Engineering, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH 44691 Abstract Medical gloves assist with the prevention of the spread of germs and are required to be worn when working with blood, body tissues, mucous membrane, and broken skin. They should provide good protection between hands and bio hazardous fluids to prevent contamination and promote safety of all healthcare workers and patients. Hence, it is critical for medical gloves to meet a standard performance criterion. Under ASTM D 3577 and D 3578, medical gloves have requirements they must meet in order to be sold and distributed as new gloves. However, there are no industry standards for gloves to meet while in use. The goal of this project was to develop a standard test for glove durability after their initial use. For the tests, gloves were worn by a mechanized prosthetic hand that was put under different conditions which are normally experienced by professionals in the medical field. This design was developed and later confirmed by Dr. Katrina Cornish: Design: Effects of media outside the glove Phosphate buffered saline (PBS), 70% ethanol, air Gloves Tested: Chloroprene, Latex 2, Nitrile, Polyvinyl Chloride and Latex 1 To evaluate glove durability during use, the gloves were immersed in different media before being subjected to contact with an abrasive surface. From this design, ranking of commercially available gloves are developed based on the time until failure of the gloves in these tests. -

Hydrogen and Fuel Cells in Japan
HYDROGEN AND FUEL CELLS IN JAPAN JONATHAN ARIAS Tokyo, October 2019 EU-Japan Centre for Industrial Cooperation ABOUT THE AUTHOR Jonathan Arias is a Mining Engineer (Energy and Combustibles) with an Executive Master in Renewable Energies and a Master in Occupational Health and Safety Management. He has fourteen years of international work experience in the energy field, with several publications, and more than a year working in Japan as an energy consultant. He is passionate about renewable energies, energy transition technologies, electric and fuel cell vehicles, and sustainability. He also published a report about “Solar Energy, Energy Storage and Virtual Power Plants in Japan” that can be considered the first part of this document and is available in https://lnkd.in/ff8Fc3S. He can be reached on LinkedIn and at [email protected]. ABOUT THE EU-JAPAN CENTRE FOR INDUSTRIAL COOPERATION The EU-Japan Centre for Industrial Cooperation (http://www.eu-japan.eu/) is a unique venture between the European Commission and the Japanese Government. It is a non-profit organisation established as an affiliate of the Institute of International Studies and Training (https://www.iist.or.jp/en/). It aims at promoting all forms of industrial, trade and investment cooperation between the EU and Japan and at improving EU and Japanese companies’ competitiveness and cooperation by facilitating exchanges of experience and know-how between EU and Japanese businesses. (c) Iwatani Corporation kindly allowed the use of the image on the title page in this document. Table of Contents Table of Contents ......................................................................................................................... I List of Figures ............................................................................................................................ III List of Tables .............................................................................................................................. -

Chemical Resistance List
Chemical Resistance List Resistance Substance Permeation Time/Level to Degradation Fluoro- natural chloro- nitrile/ nitrile carbon butyl latex prene chloroprene rubber NR CR CR NBR FKM IIR NR NR CR CR NBR NBR NBR NBR NBR FKM IIR IIR NBR NBR 395 450, 451 720, 722 717 727 730, 732 740, 741 743 754 764 890 897 898 chemical physical 403 706 723, 725 733, 836 742, 757 state 708 726 736 - 739 759 - 0 - 0 0 + 1-methoxy-2-propanol paste 4 2 2 3 4 4 B 1 3 4 6 6 - 0 - 0 0 + 1-methoxy-2-propyl acetate liquid 3 1 1 3 3 A B 2 3 6 6 - 0 0 - 0 + 1-methyl-2-pyrrolidone liquid 5 2 3 3 3 2 A B 1 3 3 6 6 - 0 + + + - 1,1,2-trichlorotrifluoroethane liquid 1 0 5 4 6 6 1 1 2 1 6 1 2 - - - - - - 1.2-epoxy ethane (ethylene oxide) liquid B A A A A 0 0 0 B 1 2 - - - - - - 1.2-epoxy propane (propylene oxide) liquid B A A A 1 A 0 0 0 B 1 2 + + + + + + 1.2-propanediol liquid 6 6 6 6 6 6 6 6 6 6 6 6 6 - + - + + 0 2-ethyl hexyl acrylate liquid 2 1 1 5 6 1 1 2 6 2 3 - 0 0 0 + + 2-mercaptoethanol liquid 3 2 4 4 4 4 1 1 3 6 6 6 - - - 0 0 - 2-methoxy-2-methyl propane liquid 1 B B 2 4 A 1 4 1 3 2 2 - - - - - 0 3-hexanone liquid 1 B 1 1 1 0 0 0 0 0 3 3 - - - - - 0 4-heptanone liquid 1 A 1 1 1 A 0 0 0 B 3 3 - - - - - + acetaldehyde liquid 1 1 1 1 B 0 0 0 A 0 6 6 0 0 0 - - + acetic acid anhydride liquid 6 3 3 3 3 2 A B 1 B 2 6 6 + + + + + + acetic acid, 10 % liquid 6 6 6 6 6 6 6 6 6 6 6 6 6 0 + + + + + acetic acid, 50 % liquid 5 4 6 6 6 2 4 6 6 6 6 - - - - 0 + acetic acid, conc. -

Download Author Version (PDF)
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 10Journal Name RSC Advances Dynamic Article Links ► Cite this: DOI: 10.1039/c0xx00000x www.rsc.org/xxxxxx ARTICLE TYPE Reverse Iodine Transfer Polymerization (RITP) of Chloroprene Jia Hui, Yan Shi,* Tao Li, Jie Wu and Zhifeng Fu Received (in XXX, XXX) Xth XXXXXXXXX 20XX, Accepted Xth XXXXXXXXX 20XX DOI: 10.1039/b000000x 5 State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China E-mail: [email protected] Fax: +86-010-64423811; Tel: +86-010-64416783 10 Reversible-deactivation radical polymerization (RDRP) of chloroprene (2-chloro-1,3-butadiene, CP) using reverse iodine transfer polymerization (RITP) has been demonstrated for the first time. -
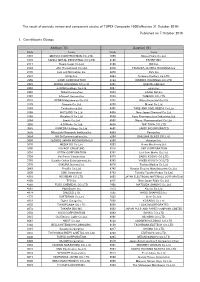
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

Vinyl Chloride Production
Vinyl Chloride Production Capstone Design Project Spring 2003 Chemical Engineering -University of Oklahoma Jeremy Dry Bryce Lawson Phuong Le Israel Osisanya Deepa Patel Anecia Shelton Vinyl Chloride Production Plant Table of Contents Section 1: Introduction............................................................................................5 Section 2: Available Processes ................................................................................5 2.1 Vinyl Chloride from Acetylene ...............................................................5 2.2 Vinyl Chloride from Ethane.....................................................................6 2.3 Vinyl Chloride from Ethylene .................................................................6 Section 3: Process Design ........................................................................................7 3.1 Thermodynamics......................................................................................7 3.2 Balanced Process Overview ....................................................................7 3.3 Balance Process Outline ..........................................................................7 3.4 Direct Chlorination Reactor Design.........................................................8 3.5 Direct Chlorination Process Simulation...................................................9 3.6 Direct Chlorination Control and Instrumentation....................................10 3.7 Oxychlorination Reactor Design..............................................................10 -

Notice of the 96Th Annual General Meeting of Shareholders
(Note) This is a translation of the official Japanese original for reference purposes only. In the event of any discrepancy between this translation and the official Japanese original, the Japanese original shall prevail. Please note that differences between this translation and those in the previous years may not necessarily mean that there have been changes in the official Japanese original, since the translation differences may stem only from a more accurate translation. [Security Code: 7011] June 7, 2021 To the Shareholders: Seiji Izumisawa, President and CEO Mitsubishi Heavy Industries, Ltd. 2-3, Marunouchi 3-chome, Chiyoda-ku, Tokyo NOTICE OF THE 96TH ANNUAL GENERAL MEETING OF SHAREHOLDERS We are pleased to announce that the 96th Annual General Meeting of Shareholders of Mitsubishi Heavy Industries, Ltd. (“MHI”) will be held as described below. With a view to preventing further spread of novel coronavirus disease (COVID-19), we request that you forgo visiting the General Meeting of Shareholders venue on the day. Please examine the Reference Materials Relating to the General Meeting of Shareholders (pages 5- 29), and exercise your voting rights in advance as indicated in the “Instructions for voting,” on pages 3-4, by 5:30 p.m. on Monday, June 28, 2021 (Japan time). 1. Date and Time: Tuesday, June 29, 2021 at 10:00 a.m. (Japan time) 2. Place: Tokyo Kaikan, 3F “Rose” 2-1, Marunouchi 3-chome, Chiyoda-ku, Tokyo 1 3. Purposes: To report on the following items: Item No. 1: Business Report, Consolidated Financial Statements for the 2020 fiscal year (from April 1, 2020 to March 31, 2021), and Audit Report on the Consolidated Financial Statements by the Financial Auditor and Audit and Supervisory Committee. -

For Investors 2017 Annual Report
SHOWA DENKO K.K. ANNUAL REPORT 2017 Annual Report 2017 Showa Denko K.K. We at the Showa Denko Group will provide products and services that are useful and safe and exceed our customers’ expectations, thereby enhancing the value of the Group, giving satisfaction to our shareholders, and contributing to the sound growth of international society Our Vision as a responsible corporate citizen. Profile Showa Denko at a Glance Ranked as one of Japan’s leading chemical companies, Showa Denko K.K. (SDK) operates in six major segments: petrochemicals, chemicals, electronics, inorganics, Net sales 2017 16.0% aluminum, and others. 30.1% The Showa Denko Group has been proceeding with its medium-term business ¥780.4 12.6% plan “Project 2020+” since 2016. Under this business plan, the Group will expand and strengthen “Individualized businesses,” which are expected to maintain their billion high-level profitability and stability, and promote these businesses in the global Note: The ratios for segments have 8.8% been calculated after adding % market. The Group will enhance the capability to resist fluctuations in market prices the amount of adjustments to 17.8 net sales. 14.7% by providing customers with attractive products and services. Moreover, the Group will reform its business model, and improve existing businesses’ earning power. Thus, the Group will enhance the corporate value. Petrochemicals In the business portfolio we aim to realize under “Project 2020+,” we classified Olefins (ethylene and propylene), polymer (polypropylene), and organic chemicals (vinyl businesses into four categories: “Growth-accelerating,” “Advantage-establishing,” acetate monomer, ethyl acetate, and allyl “Base-shaping,” and “Rebuilding.” We defined missions for each business category alcohol) in order to strengthen our businesses. -

3,4-Dichlorobut-1-Ene Cas N°: 760-23-6
OECD SIDS 3,4-DICHLOROBUT-1-ENE FOREWORD INTRODUCTION 3,4-DICHLOROBUT-1-ENE CAS N°: 760-23-6 UNEP PUBLICATIONS OECD SIDS 3,4-DICHLOROBUT-1-ENE SIDS INITIAL ASSESSMENT PROFILE CAS No. 760-23-6 Chemical Name 3,4-Dichlorobut-1-ene Structural Formula CH2=CH-CHCl-CH2Cl RECOMMENDATIONS The chemical is a candidate for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Oral LD50 and inhalation LC50 of 3,4-dichlorobut-1-ene (3,4-DCB) are about 940 mg/kg and 2100 ppm, respectively. Inhalation repeated dose study in rats conducted for 14 days, 6 hours/day, 5 days/week at doses of 104 mg/m3 (20 ppm) and 1037 mg/m3 (200 ppm) of 3,4-DCB. Relative liver weight increased and change in liver cell morphology was observed at 1037 mg/m3 dose. This chemical is slightly irritating to skin and eyes. Acute skin irritation in rabbits according to OECD TG 404 causes erythema but does not cause systemic intolerance reaction. Acute eye irritation study by instillation into the conjunctival sac of rabbits according to OECD TG 405 causes corneal opacity and conjunctival redness but does not cause systemic intolerance reaction. In an oral study in rats by OECD combined repeated dose and reproductive/developmental toxicity screening test [OECD TG 422] at doses of 0, 0.4, 2, 10 and 50 mg/kg/day for at least 44 days, organ weight and histopathological changes were induced. In males, absolute kidney weights were slightly increased with 10 mg/kg and absolute and relative weights of the liver and kidneys were increased with 50 mg/kg.