Evaluation of Recent Developments Regarding Alternative Thermal Waste Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acquisition of Printed Wiring Board Business of Showa Denko Materials Co., Ltd
Polaris Capital Group Co., Ltd. June 7, 2021 Acquisition of Printed Wiring Board Business of Showa Denko Materials Co., Ltd. and Its Subsidiaries Polaris Private Equity Fund V, L.P., etc., managed by Polaris Capital Group Co., Ltd. (“Polaris”) have recently signed an agreement with Showa Denko Materials Co., Ltd. (“Showa Denko Materials”) to acquire all the shares outstanding of a new company (the “Newly-established Company”) established to succeed Printed Wiring Board (“PWB”) business operated by Showa Denko Materials and its subsidiaries (the “Target Business”). Based on its excellence in R&D and technical capabilities developed through its more than 50 years of operations in the global PWB market, Target Business offers a wide range of products with unique strengths, including ultra-high-density Multi Wiring Boards (“MWB”) (used for probe card for high- end DRAM testing) holding a dominant market share, high precision module PWB (used for RF module for 5G smartphone, etc.), Multilayer printed wiring Boards (“MLB”) for high-speed application (used for 400Gbps switch for data center, etc.), and aluminum-based PWB (used for automotive LED lighting, etc.). Target Business has won high customer satisfaction and therefore built strong relationships with leading players in various industries, leveraging its high yield rate and low defect rate, which are essential for winning in the high-end markets. Target Business maintains the leading position in the global PWB market backed by five manufacturing sites in Japan and Singapore, each of which -
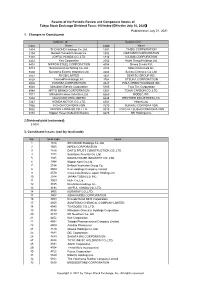
Published on July 21, 2021 1. Changes in Constituents 2
Results of the Periodic Review and Component Stocks of Tokyo Stock Exchange Dividend Focus 100 Index (Effective July 30, 2021) Published on July 21, 2021 1. Changes in Constituents Addition(18) Deletion(18) CodeName Code Name 1414SHO-BOND Holdings Co.,Ltd. 1801 TAISEI CORPORATION 2154BeNext-Yumeshin Group Co. 1802 OBAYASHI CORPORATION 3191JOYFUL HONDA CO.,LTD. 1812 KAJIMA CORPORATION 4452Kao Corporation 2502 Asahi Group Holdings,Ltd. 5401NIPPON STEEL CORPORATION 4004 Showa Denko K.K. 5713Sumitomo Metal Mining Co.,Ltd. 4183 Mitsui Chemicals,Inc. 5802Sumitomo Electric Industries,Ltd. 4204 Sekisui Chemical Co.,Ltd. 5851RYOBI LIMITED 4324 DENTSU GROUP INC. 6028TechnoPro Holdings,Inc. 4768 OTSUKA CORPORATION 6502TOSHIBA CORPORATION 4927 POLA ORBIS HOLDINGS INC. 6503Mitsubishi Electric Corporation 5105 Toyo Tire Corporation 6988NITTO DENKO CORPORATION 5301 TOKAI CARBON CO.,LTD. 7011Mitsubishi Heavy Industries,Ltd. 6269 MODEC,INC. 7202ISUZU MOTORS LIMITED 6448 BROTHER INDUSTRIES,LTD. 7267HONDA MOTOR CO.,LTD. 6501 Hitachi,Ltd. 7956PIGEON CORPORATION 7270 SUBARU CORPORATION 9062NIPPON EXPRESS CO.,LTD. 8015 TOYOTA TSUSHO CORPORATION 9101Nippon Yusen Kabushiki Kaisha 8473 SBI Holdings,Inc. 2.Dividend yield (estimated) 3.50% 3. Constituent Issues (sort by local code) No. local code name 1 1414 SHO-BOND Holdings Co.,Ltd. 2 1605 INPEX CORPORATION 3 1878 DAITO TRUST CONSTRUCTION CO.,LTD. 4 1911 Sumitomo Forestry Co.,Ltd. 5 1925 DAIWA HOUSE INDUSTRY CO.,LTD. 6 1954 Nippon Koei Co.,Ltd. 7 2154 BeNext-Yumeshin Group Co. 8 2503 Kirin Holdings Company,Limited 9 2579 Coca-Cola Bottlers Japan Holdings Inc. 10 2914 JAPAN TOBACCO INC. 11 3003 Hulic Co.,Ltd. 12 3105 Nisshinbo Holdings Inc. 13 3191 JOYFUL HONDA CO.,LTD. -

Highlights of Major General Chemical Manufacturers' Financial Results for Fiscal Year Ended March 2021
21-D-0223 June 7, 2021 Highlights of Major General Chemical Manufacturers’ Financial Results for Fiscal Year Ended March 2021 The following are Japan Credit Rating Agency, Ltd. (JCR)’s perception of the current status and highlights for rating concerning the financial results for the fiscal year ended March 2021 (FY2020) and earnings forecasts for FY2021 of Japan’s 7 general chemical manufacturers: ASAHI KASEI CORPORATION, Showa Denko K.K. (with January-December accounting period), SUMITOMO CHEMICAL COMPANY, LIMITED, TOSOH CORPORATION, Mitsui Chemicals, Inc., Mitsubishi Chemical Holdings Corporation (“Mitsubishi Chemical HD”) and Ube Industries, Ltd. 1. Industry Trend In recent years, business environment of chemical industry has been somewhat severe. Since 2018, trade friction between the U.S. and China has been a negative factor, and in 2020, the COVID-19 pandemic broke out. The COVID-19 pandemic has had a significant negative impact on social and economic activities around the world. Although uncertainty about the future of economic trend has lessened compared to the time when the pandemic broke out, the containment cannot still be foreseen. With regard to bulk chemicals, average utilization rate of domestic ethylene centers in fiscal 2020 was 93.9% (compared to an average of 94.2% in fiscal 2019), falling below the full utilization level of 95% for the second consecutive year. However, the monthly capacity utilization rate hovered around 90% in the first half of the fiscal year, but began to pick up in the second half, with some months exceeding 95%. In addition to recoveries in consumer activity and demand for downstream products, recent cold wave in the U.S. -

Hydrogen and Fuel Cells in Japan
HYDROGEN AND FUEL CELLS IN JAPAN JONATHAN ARIAS Tokyo, October 2019 EU-Japan Centre for Industrial Cooperation ABOUT THE AUTHOR Jonathan Arias is a Mining Engineer (Energy and Combustibles) with an Executive Master in Renewable Energies and a Master in Occupational Health and Safety Management. He has fourteen years of international work experience in the energy field, with several publications, and more than a year working in Japan as an energy consultant. He is passionate about renewable energies, energy transition technologies, electric and fuel cell vehicles, and sustainability. He also published a report about “Solar Energy, Energy Storage and Virtual Power Plants in Japan” that can be considered the first part of this document and is available in https://lnkd.in/ff8Fc3S. He can be reached on LinkedIn and at [email protected]. ABOUT THE EU-JAPAN CENTRE FOR INDUSTRIAL COOPERATION The EU-Japan Centre for Industrial Cooperation (http://www.eu-japan.eu/) is a unique venture between the European Commission and the Japanese Government. It is a non-profit organisation established as an affiliate of the Institute of International Studies and Training (https://www.iist.or.jp/en/). It aims at promoting all forms of industrial, trade and investment cooperation between the EU and Japan and at improving EU and Japanese companies’ competitiveness and cooperation by facilitating exchanges of experience and know-how between EU and Japanese businesses. (c) Iwatani Corporation kindly allowed the use of the image on the title page in this document. Table of Contents Table of Contents ......................................................................................................................... I List of Figures ............................................................................................................................ III List of Tables .............................................................................................................................. -
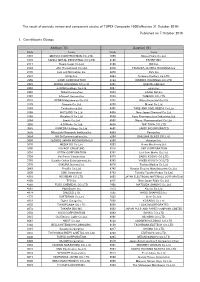
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

Notice of the 96Th Annual General Meeting of Shareholders
(Note) This is a translation of the official Japanese original for reference purposes only. In the event of any discrepancy between this translation and the official Japanese original, the Japanese original shall prevail. Please note that differences between this translation and those in the previous years may not necessarily mean that there have been changes in the official Japanese original, since the translation differences may stem only from a more accurate translation. [Security Code: 7011] June 7, 2021 To the Shareholders: Seiji Izumisawa, President and CEO Mitsubishi Heavy Industries, Ltd. 2-3, Marunouchi 3-chome, Chiyoda-ku, Tokyo NOTICE OF THE 96TH ANNUAL GENERAL MEETING OF SHAREHOLDERS We are pleased to announce that the 96th Annual General Meeting of Shareholders of Mitsubishi Heavy Industries, Ltd. (“MHI”) will be held as described below. With a view to preventing further spread of novel coronavirus disease (COVID-19), we request that you forgo visiting the General Meeting of Shareholders venue on the day. Please examine the Reference Materials Relating to the General Meeting of Shareholders (pages 5- 29), and exercise your voting rights in advance as indicated in the “Instructions for voting,” on pages 3-4, by 5:30 p.m. on Monday, June 28, 2021 (Japan time). 1. Date and Time: Tuesday, June 29, 2021 at 10:00 a.m. (Japan time) 2. Place: Tokyo Kaikan, 3F “Rose” 2-1, Marunouchi 3-chome, Chiyoda-ku, Tokyo 1 3. Purposes: To report on the following items: Item No. 1: Business Report, Consolidated Financial Statements for the 2020 fiscal year (from April 1, 2020 to March 31, 2021), and Audit Report on the Consolidated Financial Statements by the Financial Auditor and Audit and Supervisory Committee. -

For Investors 2017 Annual Report
SHOWA DENKO K.K. ANNUAL REPORT 2017 Annual Report 2017 Showa Denko K.K. We at the Showa Denko Group will provide products and services that are useful and safe and exceed our customers’ expectations, thereby enhancing the value of the Group, giving satisfaction to our shareholders, and contributing to the sound growth of international society Our Vision as a responsible corporate citizen. Profile Showa Denko at a Glance Ranked as one of Japan’s leading chemical companies, Showa Denko K.K. (SDK) operates in six major segments: petrochemicals, chemicals, electronics, inorganics, Net sales 2017 16.0% aluminum, and others. 30.1% The Showa Denko Group has been proceeding with its medium-term business ¥780.4 12.6% plan “Project 2020+” since 2016. Under this business plan, the Group will expand and strengthen “Individualized businesses,” which are expected to maintain their billion high-level profitability and stability, and promote these businesses in the global Note: The ratios for segments have 8.8% been calculated after adding % market. The Group will enhance the capability to resist fluctuations in market prices the amount of adjustments to 17.8 net sales. 14.7% by providing customers with attractive products and services. Moreover, the Group will reform its business model, and improve existing businesses’ earning power. Thus, the Group will enhance the corporate value. Petrochemicals In the business portfolio we aim to realize under “Project 2020+,” we classified Olefins (ethylene and propylene), polymer (polypropylene), and organic chemicals (vinyl businesses into four categories: “Growth-accelerating,” “Advantage-establishing,” acetate monomer, ethyl acetate, and allyl “Base-shaping,” and “Rebuilding.” We defined missions for each business category alcohol) in order to strengthen our businesses. -

SHOWA DENKO K.K. August 12, 2020
We “Act” to touch the heart and make society better Second Quarter, 2020 Financial Results - Consolidated - SHOWA DENKO K.K. August 12, 2020 Motohiro Takeuchi, CFO Representative Director & Managing Corporate Officer Performance forecast and other statements pertaining to the future as contained in this presentation are based on the information available as of today and assumptions as of today regarding risk factors that could affect our future performance. Actual results may differ materially from the forecast due to a variety of risk factors, including, but not limited to, the influence of the coronavirus disease 2019 (COVID-19) on the world economy, the economic conditions, costs of naphtha and other raw materials, demand for our products such as graphite electrodes and other commodities, market conditions, and foreign exchange rates. We undertake no obligation to update the forward-looking statements unless required by law. Consolidated Companies Consolidated subsidiaries: 152 (newly consolidated: 91 companies related to Hitachi Chemical) (Showa Denko Materials segment) -Major companies Hitachi Chemical Co., Ltd. Hitachi Chemical Electronic Hitachi Chemical (Johor) Sdn. Bhd. SD (Shanghai) Co., Ltd. Materials (Guangzhou) Ltd. Hitachi Chemical Asia (Thailand) Co., Ltd. Hitachi Chemical (Nantong) Hitachi Chemical (Suzhou) Co., Ltd. Hitachi Chemical Co. America, Ltd. Co., Ltd. Hitachi Chemical (Dongguan) Co., Ltd. Hitachi Powdered Metals (USA), Inc. PT Hitachi Chemical Indonesia FIAMM Energy Technology S.p.A. Equity method applied: -

Press Release
Press Release Press contact Corporate Communications Office Tel: +81-3-3457-2105 1-1-1 Shibaura, Minato-ku, Tokyo 105-8001, Japan Fax: +81-3-5444-9202 URL: http://www.toshiba.co.jp/about/press/index.htm FOR IMMEDIATE RELEASE July 7, 2011 Toshiba establishes new HDD technology centers for future generation high density recordings Strengthen advanced cutting edge technologies and manufacturing technologies TOKYO—Toshiba Corporation (TOKYO: 6502) today announced that it will establish two new technology development centers for hard disk drives (HDD). The “HDD Advanced Technology Center” will seek to accelerate development of higher density technology and the “HDD Manufacturing Technology Center” will develop enhanced manufacturing capabilities. Both will open on July 16th. Toshiba will cooperate in the development work with TDK Corporation, which manufactures magnetic heads, and Showa Denko K.K., a disk media manufacturer. In 2004, Toshiba led the industry in introducing perpendicular magnetic recording technology (PMR), now the dominant technology for high areal density HDD, and in doing so realized significant advances in hard disk drive capacity. However the physical limit of PMR is widely recognized at around 1.6Gbit/mm2 (1Tbit/in2) and densities beyond that will require further technology breakthroughs. Toshiba, in cooperation with TDK and Showa Denko, is establishing an HDD Advanced Technology Center to focus on innovation in future generations of higher capacity HDD by investigating promising technologies, including energy-assisted technologies and bit pattern technology. By establishing a complementary manufacturing technology development center at the same time, Toshiba, in cooperation with TDK, expects to develop stable manufacturing methods for future cutting-edge HDDs and to achieve the very earliest launch of differentiated products. -

SHOWA DENKO Report 2018Download
Contact desk Public Relation Office, Showa Denko K.K. TEL : +81-3-5470-3235 Website : http : //www.sdk.co.jp/english/csr.html E-Mail : [email protected] We adopted eco-friendly paper and printing. SHOWA DENKO REPORT SRI indexes adopting Showa Denko As of January 2018 2018 Our Vision CONTENTS Showa Denko Group, under its Vision, has strived to realize “a company contributing to the sound growth of society” that About Showa Denko 44 Highlights of CSR Activities contributes toward creating a society where affluence and sustainability are harmonized. 48 Responsible Care In order to be trusted and evaluated by society through the continuous improvement of the corporate value, it is important 04 Showa Denko Group in Numbers 60 CSR procurement to maintain and develop appropriate relations with stakeholders such as shareholders, customers, business partners, local 06 Showa Denko’s Ties with Society communities and employees. 62 Human Rights and Labor Practice 08 Message from CEO Also, in order to realize our Vision and develop the Group sustainably, we have defined what we should do as the “Our Code of 66 Performance Data 10 Showa Denko’s Business Model Conduct.” 68 Third-party Verification 12 Medium-term business plan, "Project 2020+" We clarify this as "Our Vision" and promote the management for its realization. Financial We have established our slogan as a declaration to firmly “promise” our stakeholders this policy. 14 Introduction of Businesses of the Group 20 Dialogue between the CEO and Outside Expert 70 Message from CFO 23 R&D of the Showa Denko Group 71 Financial Highlights Governance 72 Management’s Discussion and Analysis 76 Consolidated financial statements 30 Corporate Governance Our 82 Corporate Data Vision CSR Our Slogan 38 CSR Strategy “Shaping Ideas” 42 Stakeholder communications Our Code of Conduct Editorial policies Report scope Its Practical Guide The Showa Denko Group restructured its CSR report and annual report in 2017 Period covered by this Report to publish the Showa Denko Report as an integrated report. -

Japan Forum 2016 23-26 February 2016
Japan Forum 2016 23-26 February 2016 . Grand Hyatt Tokyo Registration Fortune favours the brave th wales.clsa.com with Please come join us at the 13 CLSA Japan Forum to be held from 23 (Tue) to your CLSA delegate ID 26 (Fri) February at the Grand Hyatt Tokyo. Marching confidently into its 13th year, the CLSA Japan Forum is the country's most comprehensive annual investment event. In 2015, 650 investors from 22 countries met with the CEOs and CFOs of 177 listed companies. Delegates also heard from over 40 specialist guests and keynote speakers, who shared th their unique insights on a broad range of topics. Please come to the 13 CLSA Japan Forum for direct access to another all-encompassing ensemble of key decision-makers and global opinion leaders. It has been a white-knuckle ride in the Japanese stock market this year, with a collapse in the market at the start of the year on China slowdown fears, compounded by unease caused by the Fed rate hike decision. Then, at the end of January, the market was shaken out of its panic by an extraordinary decision by the BoJ to charge a penalty rate on banks’ excess reserves - in addition to roaring QE at 16% of GDP per year that it was already doing. Will it work, and who will be the winners and losers? The pivotal event of the year will be the elections, which have to be held by the end of July. Before them, Abe has to juice the economy and be popular: will he give way to temptation and announce pushout of his promised April 2017 VAT hike? It would certainly be popular with voters. -

Product Change Notification
Cypress Semiconductor Corporation – An Infineon Technologies Company 198 Champion Court, San Jose, CA 95134. Tel: (408) 943-2600 PRODUCT CHANGE NOTIFICATION PCN: PCN210604 Date: February 14, 2021 Subject: Transfer of Assembly Operations to Greatek Electronics Inc. for Select 32-Lead TSOP I Package Automotive Memory Products To: PCN ALERTS MOUSER [email protected] Change Type: Major Description of Change: Cypress announces the qualification of Greatek Electronics Inc., Taiwan located at No. 136, Gong-Yi Rd., Zhunan Township, Miaoli County 350, Taiwan, as an alternate assembly site for select automotive Memory products offered in 32-Lead TSOP I package. These products are currently process at Jiangsu Changjiang Electronics Technology Co., Ltd (JCET) of Cypress’ subcontractor in China; and Orient Semiconductor Electronics (OSET) of Cypress’ subcontractor in Taiwan. The transfer of assembly operations to Greatek is motivated by JCET’s phasing out (i.e., End-Of-Life) of TSOP manufacturing operations, as previously announced in advance PCN - APCN 201002 and also as alternative assembly site. Given the imminent phase out of operations at JCET, and the dynamically changing market conditions, Cypress is pleased to offer supply of changed material (i.e., Greatek assembled product) ahead of the implementation date. Customers are strongly encouraged to avail of this option, where production volumes of Greatek assembled product can be secured and shipped against current orders. Please contact your Cypress Sales Representative for more information on availing this option. Greatek is certified by international quality and safety standards, namely, ISO 9001, IATF 16949, ISO 14001, and ISO 26262. These certificates, along with their Sony Green Partnership certificate, can be viewed on their corporate web site: http://www.greatek.com.tw/ Document No.