General Disclaimer One Or More of the Following Statements May Affect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Disclaimer One Or More of the Following Statements May Affect
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) X-640-10-365 PREPRINT NASA TM X16 PHYSICAL CHEMISTRY OF THE AOUELLOUL CRATER GLASS JOHN A. O'KEEFE r- oCr j OCTOBER 1970 GODDARD SPACE FLIGHT CENTER GREENBELT, MARYLAND A • N (ACCESSION NUM ER (T U) o^c O TM ( G (C DE) C (NASA CR OR TMX OR ADNUMBER) (CATEGORY) PHYSICAL CHEMISTRY OF THE AOUELLOUL CRATER GLASS John A. O'Keefe Laboratory for Space Physics Goddard Space Flight Center Greenbelt, Maryland z s - a ^.l i' ABSTRACT Aouelloul crater glass is studied from the point of view of the hypothesis that it is formed by impact on the local (Zli) sandstone. It is noted that the chemical analyses of the two materials do not agree in a satisfactory way even if the most significant discrepancies, namely those in Fe and H 2O are overlooked. -

Meteorite Impacts, Earth, and the Solar System
Traces of Catastrophe A Handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures Bevan M. French Research Collaborator Department of Mineral Sciences, MRC-119 Smithsonian Institution Washington DC 20560 LPI Contribution No. 954 i Copyright © 1998 by LUNAR AND PLANETARY INSTITUTE The Institute is operated by the Universities Space Research Association under Contract No. NASW-4574 with the National Aeronautics and Space Administration. Material in this volume may be copied without restraint for library, abstract service, education, or personal research purposes; however, republication of any portion thereof requires the written permission of the Insti- tute as well as the appropriate acknowledgment of this publication. Figures 3.1, 3.2, and 3.5 used by permission of the publisher, Oxford University Press, Inc. Figures 3.13, 4.16, 4.28, 4.32, and 4.33 used by permission of the publisher, Springer-Verlag. Figure 4.25 used by permission of the publisher, Yale University. Figure 5.1 used by permission of the publisher, Geological Society of America. See individual captions for reference citations. This volume may be cited as French B. M. (1998) Traces of Catastrophe:A Handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures. LPI Contribution No. 954, Lunar and Planetary Institute, Houston. 120 pp. This volume is distributed by ORDER DEPARTMENT Lunar and Planetary Institute 3600 Bay Area Boulevard Houston TX 77058-1113, USA Phone:281-486-2172 Fax:281-486-2186 E-mail:[email protected] Mail order requestors will be invoiced for the cost of shipping and handling. Cover Art.“One Minute After the End of the Cretaceous.” This artist’s view shows the ancestral Gulf of Mexico near the present Yucatán peninsula as it was 65 m.y. -

References 111 References
References 111 References Ahrens T. J. (1993) Impact erosion of terrestrial planetary atmos- Bischoff A. and Stöffler D. (1984) Chemical and structural changes pheres. Annu. Rev. Earth Planet. Sci., 21, 525–555. induced by thermal annealing of shocked feldspar inclusions Ahrens T. J. and O’Keefe J. D. (1977) Equations of state and in impact melt rocks from Lappajärvi Crater, Finland. Proc. impact-induced shock-wave attenuation on the Moon. In Lunar Planet. Sci. Conf. 14th, in J. Geophys. Res., 89, B645– Impact and Explosion Cratering: Planetary and Terrestrial B656. Implications (D. J. Roddy, R. O. Pepin, and R. B. Merrill, Bischoff L. and Oskierski W. (1987) Fractures, pseudotachylite eds.), pp. 639–656. Pergamon, New York. veins, and breccia dikes in the crater floor of the Rochechouart Aldersey-Williams H. (1995) The Most Beautiful Molecule: The impact structure, SW-France, as indicators of crater-forming Discovery of the Buckyball. John Wiley and Sons, New York. processes. In Research in Terrestrial Impact Structures ( J. Pohl, 340 pp. ed.), pp. 5–29. Earth Evolution Sciences, Intl. Mono. Ser., Alexopoulos J. S., Grieve R. A. F., and Robertson P. B. (1988) Friedr. Vieweg and Son, Wiesbaden, Germany. Microscopic lamellar deformation features in quartz: Dis- Bloss F. D. (1981) The Spindle Stage — Principles and Practice. criminative characteristics of shock-generated varieties. Cambridge Univ., New York. 340 pp. Geology, 16, 796–799. Bohor B. F. and Glass B. P. (1995) Origin and diagenesis of K/T Alvarez L. W., Alvarez W., Asaro F., and Michel H. V. (1980) impact spherules — From Haiti to Wyoming and beyond. -

Mineralógica Petrographica SHOCK METAMORPHISM AT
Acta Mineralogica-Petrographica, Szeged 2008, Vol. 48, pp. 17-31. ACTA Mineralógica Petrographica SHOCK METAMORPHISM AT TERRESTRIAL IMPACT STRUCTURES: MINERALOGICAL AND GEOLOGICAL CONSEQUENCES ARNOLD GUCSIK Max Planck Institute for Chemistry, Department of Geochemistry, Joh.-J.-Becherweg 27., D-55128, Mainz, Germany, e-mail: [email protected] ABSTRACT The impact cratering as a leading process in the formation of the planetary bodies and surfaces and their geological as well as mineralogical consequences have been summarized in this review article, which is based on PhD. thesis of Arnold Gucsik at Univeristy of Vienna. The purpose of this study is to provide the most important lithological and shock diagnostic features of shock metamorphism accompanied with terrestrial impact structures. The first section of this study gives a brief summary of the formation mechanism and stages of an impact structure as well as a short description of basics of the sock wave physics of an impact event. The next section deals with the types of terrestrial impact structures. The lithological shock-metamorphic indicators and diagnostic shock features in the target rocks are mentioned in the following sections. Key words: shock metamorphism, impact crater, shock wave, impact-derived glasses, shock-induced microdeformations INTRODUCTION years through volcanism, earthquakes, tectonic processes, Shock metamorphism is the sum of irreversible and heat flow. chemical, mineralogical and physical changes in the target • The energy is released in an impact event shattering, materials that occur during the hypervelocity impact event deforming, melting, and even vaporising large volumes of (Melosh 1989). The following chapters have been target rock in a fraction of seconds. -

African Meteorite Impact Craters: Characteristics and Geological Importance
Journal of African Earth Sciences, Vol. 18, No. 4, pp. 263-295, 1994 Pergamon Copyright © 1994 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0899-5362/94 $7.00 + 0.00 0899-5362(94)00044-1 African meteorite impact craters: characteristics and geological importance CHRISTIAN KOEBERLt~ 1Institute of Geochemistry, University of Vienna, Dr.-Karl-Lueger-Ring 1, A-1010 Vienna, Austria. 2Economic Geology Research Unit, Depa~ i.ment of Geology, University of the Witwatemmnd,Johannesburg 2050, South Africa. (Received 26 October 1993 : accepted 12 May 1994) Abslzact - Geologists have realized that impact cratering is the single most important surface-forming and modifying process for the other terrestrial planets and the satellites of all planets. The recognition of impact cratering as an important geological process on earth has been rather slow. However, geologists are now realizing that giant impacts have had a determining influence on the geological and biological evolution of our planet. The study of impact craters allows important conclusions, not only about the origin mid history of our solar system and its planets, but also about a fundamentally important geological process. In addition, impact craters may have a definite economic importance as some craters have been shown to contain important mineral or oil deposits. F'dtesn meteorite impact craters have so far been identified on the African continent:. Amguid (Algeria), Aomunga (Chad), Aouelloul (Mauritania), B.P. (Libya), Bosumtwi (Ghana), Highbury (Zimbabwe), Kalkkop (South Africa), Oasis (Libya), Ouarkziz (Algeria), Roter Kamm (Namibia), Saitpan (South Africa), Talemzane (Algeria), Tenoumer (Mauritania), T'm Bider (Algeria), and V~:lefort (South Africa). -
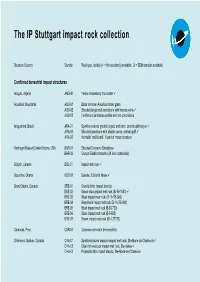
The IP Stuttgart Impact Rock Collection
The IP Stuttgart impact rock collection Structure Country Sample Rock type, locality ( = thin section(s) available; = SEM samples available) Confirmed terrestrial impact structures Amguid, Algeria AMG-01 Yellow chalcedony from crater Aouelloul, Mauritania AOU-01 Black to brown Aouelloul crater glass AOU-02 Shocked target rock sandstone with breccia veins AOU-03 Ferriferous sandstone pellets and iron concretions Araguainha, Brazil ARA-01 Spinifex-textured granitic impact melt rock, central uplift region ARA-02 Shocked sandstone with shatter cones, central uplift ARA-03 Hematite ‘melt bomb’, N part of impact structure Barringer (Meteor) Crater Arizona, USA BAR-01 Shocked Coconino Sandstone BAR-02 Canyon Diablo meteorite (IA iron octahedrite) Boltysh, Ukraine BOL-01 Impact melt rock Bosumtwi, Ghana BOS-01 Suevite, 2.5 km N Nkowi Brent Ontario, Canada BRE-01 Granitic lithic impact breccia BRE-02 Brown-black impact melt rock (Bi-59-1503) BRE-03 Black impact melt rock; (B-11475-690) BRE-04 Grey-black impact melt rock (B-11475-600) BRE-05 Black impact melt rock (B-50-720) BRE-06 Black impact melt rock (B-5-695) BRE-07 Brown impact melt rock (Bi-4-27733) Carancas, Peru CAR-01 Carancas meteorite (H-chondrite) Charlevoix Québec, Canada CHA-01 Spinifex-textured massive impact melt rock, Ste-Marie-de-Charlevoix CHA-02 Clast-rich vesicular impact melt rock, Ste-Irénée CHA-03 Polymictic lithic impact breccia, Ste-Marie-de-Charlevoix Charlevoix Québec, Canada CHA-04 Shocked and brecciated anorthosite, summit of Mont des Éboulements CHA-05 -

NURAZLIN ABDULLAH.Pdf
KAJIAN TERHADAP MINERAL POLIMORF SEBAGAI BUKTI IMPAK METEORIT DAN KAITANNYA DENGAN BAHAN ASAS INDUSTRI LITIK DI BUKIT BUNUH, LENGGONG, PERAK, MALAYSIA NURAZLIN BT ABDULLAH UNIVERSITI SAINS MALAYSIA 2017 KAJIAN TERHADAP MINERAL POLIMORF SEBAGAI BUKTI IMPAK METEORIT DAN KAITANNYA DENGAN BAHAN ASAS INDUSTRI LITIK DI BUKIT BUNUH, LENGGONG, PERAK, MALAYSIA. oleh NURAZLIN BT ABDULLAH Tesis yang diserahkan untuk memenuhi keperluan bagi Ijazah Sarjana Sastera JULAI 2017 PENGHARGAAN Syukur Alhamdulillah dengan limpah rahmat dan kesyukuran yang tidak terhingga ke hadrat Ilahi kerana dengan izin dan kekuasaanNya dapat saya menyempurnakan penulisan tesis ini. Setinggi-tinggi penghargaan dan jutaan terima kasih dirakamkan kepada Prof. Dato’ Dr. Mokhtar Saidin, Pengarah Pusat Penyelidikan Arkeologi Global (PPAG), Universiti Sains Malaysia dan selaku penyelia saya atas segala nasihat, dorongan, bantuan dan keprihatinan semasa menyempurnakan tesis ini. Pada kesempatan ini juga saya ingin merakamkan ribuan terima kasih kepada Prof. Hamzah Mohamad yang sedia memberi bimbingan dan tunjuk ajar semasa menganalisis data kajian untuk tesis ini. Sanjungan budi juga kepada semua pensyarah-pensyarah, pegawai dan staf serta rakan-rakan saya di Pusat Peyelidikan Arkeologi Global atas sokongan dan dorongan dalam menyiapkan tesis ini. Kajian ini telah disokong daripada segi kewangan terutamanya oleh Geran Penyelidikan Universiti, USM dan biasiswa Mybrain oleh Kementerian Pengajian Tinggi yang telah banyak memudahkan kajian ini. Ucapan terima kasih juga ditujukan kepada Jabatan Warisan Malaysia atas keizinan bagi memasuki kawasan kajian Bukit Bunuh. Pada masa yang sama, penghargaan dan terima kasih yang tidak terhingga ditujukan kepada keluarga tercinta. Akhir sekali, saya ingin tujukan ucapan terima kasih kepada semua yang terlibat secara langsung atau tidak langsung dalam menghasilkan disertasi ini dan diharapkan disertasi ini dapat memberikan maklumat yang berguna. -

Terrestrial Impact Structures- a Bibliography 1965-68
Terrestrial Impact Structures- A Bibliography 1965-68 By JACQUELYN H. FREEBERG GEOLOGICAL SURVEY BULLETIN 1320 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1969 UNITED STATES DEPARTMENT OF THE INTERIOR WALTER J. HICKEL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. 74-650225 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 30 cents paper cover CONTENTS Page Abstract--------------------------------------------------------- 1 Introduction______________________________________________________ 1 Seria~----------------------------------------------------------- 2 Bibliography______________________________________________________ 3· Distribution and general characteristics of impact structures_________ 3: Impact sites___________________________________________________ 12: Agnak Island Oraters _ _ __ _ __ _ __ __ _ __ __ _ _ _ __ __ _ _ __ _ _ _ __ _ _ _ __ 1Z Aouelloul Crater___________________________________________ 12 Arn Valley Craters________________________________________ 12 Barringer Crater__________________________________________ 12 Bass Strait_______________________________________________ 13 Boxhole Crater____________________________________________ 14 Brent Crater______________________________________________ 14 Butare Crater_______________________________________ ------ 14 Campo del Cielo Craters----------------------------------- 14 Carswell Lake structure _________________________ . _ _ _ __ _ _ __ _ 15 Chassenon -

Fulgurite: a Unique Mineral Formed by Impact of Lightening
Open Access e-Journal Earth Science India - www.earthscienceindia.info Popular Issue, V (IV), October, 2012, p. 1-8 Fulgurite: A unique mineral formed by impact of lightening Arun Kumar This study discloses new facts about the environment of the ancient Sahara Desert and the chemical changes during the interaction between atmosphere and lightning. It also shows that fulgurites are archives of ancient lightning, atmospheres and ecology. In June, 2012, I visited the Museum of Science in Boston, U.S.A. and saw a couple of presentations (Figures 1 and 2) about petrified lightning. In my over forty years as a petroleum geologist, stratigraphic palynologist and environmental micropaleontologist, I had never heard of fulgurite and could not imagine that a term ‘petrified lightning’ exists. However, I certainly believe that mineralogists and other well-informed geologists are more likely to be aware of this phenomenon as well as this mineral. I felt intrigued and decided to learn more about it. The display (Figure 1) at the museum reads as follows: Petrified Lightning: This rare mineral formation called the fulgurite, was produced in less than one ten- thousands of a second when lightning struck the ground near Columbia, South Carolina. The lightning bolt traveled through a thick layer of dry sand and it heated to a temperature greater than 1800 0 C (3200 0 F). The sand was instantly melted and blown aside, forming a thin, glassy tube surrounding the path of the lightning bolt. Fulgurites as long as this are rare; most are less than 30 cm (one foot) long. Lent by Mineralogical Museum Harvard University Navarro-González et al. -

Evidence from Lonar Crater, India P
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110, B12402, doi:10.1029/2005JB003662, 2005 Structural effects of meteorite impact on basalt: Evidence from Lonar crater, India P. Senthil Kumar National Geophysical Research Institute, Hyderabad, India Received 31 January 2005; revised 17 August 2005; accepted 26 August 2005; published 8 December 2005. [1] Lonar crater is a simple, bowl-shaped, near-circular impact crater in the 65 Myr old Deccan Volcanic Province in India. As Lonar crater is a rare terrestrial crater formed entirely in basalt, it provides an excellent opportunity to study the impact deformation in target basalt, which is common on the surfaces of other terrestrial planets and their satellites. The present study aims at documenting the impact deformational structures in the massive basalt well exposed on the upper crater wall, where the basalt shows upward turning of the flow sequence, resulting in a circular deformation pattern. Three fracture systems (radial, concentric, and conical fractures) are exposed on the inner crater wall. On the fracture planes, plumose structures are common. Uplift and tilting of the basalt sequence and formation of the fractures inside the crater are clearly related to the impact event and are different from the preimpact structures such as cooling-related columnar joints and fractures of possible tectonic origin, which are observed outside the crater. Slumping is common throughout the inner wall, and listric faulting displaces the flows in the northeastern inner wall. The impact structures of Lonar crater are broadly similar to those at other simple terrestrial craters in granites and clastic sedimentary rocks and even small-scale experimental craters formed in gabbro targets. -

Manfred Gottwald, Thomas Kenkmann, Wolf Uwe Reimold Verlag Dr
TERRESTRIAL IMPACT STRUCTURES: The TanDEM-X Atlas. Vol.1 and 2. Manfred Gottwald, Thomas Kenkmann, Wolf Uwe Reimold Verlag Dr. Friedrich Pfeil, München 2020, 608 p. ISBN 978-3-89937-261-8 TERRESTRIAL IMPACT STRUCTURES The TanDEM-X Atlas 1 Africa, North/Central America, South America M. Gottwald, T. Kenkmann and W. U. Reimold Verlag Dr. Friedrich Pfeil Manfred Gottwald Thomas Kenkmann Wolf Uwe Reimold TERRESTRIAL IMPACT STRUCTURES The TanDEM-X Atlas 1 Africa, North/Central America, South America Verlag Dr. Friedrich Pfeil · München 2020 ISBN 978-3-89937-261-8 Bibliografische Information Der Deutschen Bibliothek Die Deutsche Bibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Daten sind im Internet über http://dnb.dnb.de abrufbar. front cover Vredefort Dome, Meteor Crater, Serra da Cangalha (from left to right). Top row: TanDEM-X topographic maps; bottom row Sentinel-2 RGB images, fused with the TanDEM-X digital elevation model. back cover Top: Meteor Crater (photo: T. Kenkmann); bottom: B.P. structure (photo: W. U. Reimold). print support by and Wolf Uwe Reimold and Manfred Gottwald Copyright © 2020 by Verlag Dr. Friedrich Pfeil, München Alle Rechte vorbehalten – All rights reserved. Dieses Werk ist urheberrechtlich geschützt. Jede Art der Vervielfältigung und Weitergabe, auch auszugsweise und in elektronischer Form, insbesondere im Internet, bedarf der ausdrücklichen Genehmigung durch den Verlag Dr. Friedrich Pfeil. Druckvorstufe: Verlag Dr. Friedrich Pfeil, München Druck: PBtisk a.s., Prˇíbram I – Balonka ISBN 978-3-89937-261-8 Printed in the European Union Verlag Dr. Friedrich Pfeil, Wolfratshauser Straße 27, 81379 München, Germany Tel.: + 49 (0)89 5528600-0 • Fax: + 49 (0)89 5528600-4 • E-Mail: [email protected] www.pfeil-verlag.de Contents Contents Preface ..................................................................... -

Aouelloul Impact Crater, Mauritania: New Structural and Lithological Data
Large Meteorite Impacts VI 2019 (LPI Contrib. No. 2136) 5093.pdf AOUELLOUL IMPACT CRATER, MAURITANIA: NEW STRUCTURAL AND LITHOLOGICAL DATA. E. cheikh Ould Mohamed Navee1, H. Chennaoui Aoudjehane1, D. Baratoux2, L. Ferrière3, and M. S. Ould Sabar4, 1GAIA, Laboratory, Hassan II University of Casablanca, Faculty of Sciences Ain Chock Casablanca, Morocco ([email protected]), 2Geosciences Environnement Toulouse, University of Toulouse, Research Institute for Development & CNRS, 14, Avenue Edouard Belin, 31400 Toulouse, France, 3Natural History Museum, Burgring 7, A-1010 Vienna, Austria, 4Department of Geology, Faculty of Science and Technology University of Nouakchott El-Aasriya, Nouakchott, Mauritania. Introduction: The Aouelloul impact crater is lo- Bing Maps aerial image of the Aouelloul crater. The cated in Mauritania, in the Adrar region, about 50 km locations of impact glass fragments collected at several SE from the city of Atar. It was first discovered from locations around the crater are also reported, allowing the air by A. Pourquié in 1938 and visited by him on us to update the spatial distribution of the glass, which the same year and again in 1939. The crater was first was limited to a very small sector at the SE of the described in the scientific literature by Monod and crater on the map by [1]. All information were reported Pourquié in 1951 [1]. However, it was already known with the ArcGis software on the aerial image. for a very long time already from the local people who call it "Hofrath Aouelloul" (i.e., the “hole of Aouel- loul”) [1]. The crater, with a well-developed rim, is almost 400 m in diameter.