Preparation and Characterization of Water-Soluble Xylan Ethers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fighting Sand Encroachment Lessons from Mauritania Cover Photo: Mechanical Dune Stabilization: Installing Plant Matter M
ISSN 0258-6150 FAO FORESTRY PAPER 158 Fighting sand encroachment Lessons from Mauritania Cover photo: Mechanical dune stabilization: installing plant matter M. Ould Mohamed FAO FORESTRY Fighting sand encroachment PAPER Lessons from Mauritania 158 by Charles Jacques Berte Consultant with the collaboration of Moustapha Ould Mohamed and Meimine Ould Saleck Nature Conservation Directorate Ministry of the Environment and Sustainable Development of Mauritania FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2010 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. ISBN 978-92-5-106531-0 All rights reserved. FAO encourages the reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Office of Knowledge Exchange, Research and Extension, FAO, Viale delle Terme di Caracalla, 00153 Rome, Italy. -

00007647.Pdf
Nutritive evaluation of some browse plant species collected from Algerian arid rangelands by chemical analyses and in vitro gas production Bouazza L., Boufennara S., López S., Bousseboua H., Bodas R. in Chentouf M. (ed.), López-Francos A. (ed.), Bengoumi M. (ed.), Gabiña D. (ed.). Technology creation and transfer in small ruminants: roles of research, development services and farmer associations Zaragoza : CIHEAM / INRAM / FAO Options Méditerranéennes : Série A. Séminaires Méditerranéens; n. 108 2014 pages 305-310 Article available on line / Article disponible en ligne à l’adresse : -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- http://om.ciheam.org/article.php?IDPDF=00007647 -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- To cite this article / Pour citer cet article -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Bouazza L., Boufennara S., López S., Bousseboua H., Bodas R. Nutritive evaluation of some browse plant species collected from Algerian arid rangelands by chemical analyses and in vitro gas production. In : Chentouf M. (ed.), López-Francos A. (ed.), Bengoumi M. (ed.), Gabiña D. (ed.). Technology creation and transfer in small ruminants: roles of research, development -

Multiple-Factor Associative Effects of Peanut Shell Combined with Alfalfa and Concentrate Determined by in Vitro Gas Production Method
Original Paper Czech Journal of Animal Science, 64, 2019 (8): 352–360 https://doi.org/10.17221/94/2019-CJAS Multiple-factor associative effects of peanut shell combined with alfalfa and concentrate determined by in vitro gas production method Jiu Yuan1*, Xinjie Wan2 1College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, P.R. China 2Gansu Zhenghe Biotechnology Co., Ltd, Lanzhou, P.R. China *Corresponding author: [email protected] Citation: Yuan J., Wan X.J. (2019): Multiple-factor associative effects of peanut shell combined with alfalfa and concentrate determined by in vitro gas production method. Czech J. Anim. Sci., 64, 352–360. Abstract: The associative effects (AE) between concentrate (C), peanut shell (P) and alfalfa (A) were investigated by means of an automated gas production (GP) system. The C, P and A were incubated alone or as 40 : 60 : 0, 40 : 45 : 15, 40 : 30 : 30, 40 : 15 : 45, 40 : 0 : 60 and 30 : 70 : 0, 30 : 55 : 15, 30 : 40 : 30, 30 : 25 : 45, 30 : 10 : 60, 30 : 0 : 70 mixtures where the C : roughage (R) ratios were 40 : 60 and 30 : 70. Samples (0.2000 ± 0.0010 g) of single feeds or mixtures were incubated for 96 h in individual bottles (100 ml) with 30 ml of buffered rumen fluid. GP parameters were analysed using a single exponential equation. After incubation, the residues were used to determine pH, dry matter digestibility (DMD), organic matter digestibility (OMD), volatile fatty acids (VFA) and ammonia nitrogen (NH3-N) of the incubation fluid, and their single factor AE indices (SFAEI) and multiple-factors AE indices (MFAEI) were determined. -

Lost-Crops-Of-Africa-Grains.Pdf
http://www.nap.edu/catalog/2305.html We ship printed books within 1 business day; personal PDFs are available immediately. Lost Crops of Africa: Volume I: Grains Board on Science and Technology for International Development, Office of International Affairs, National Research Council ISBN: 0-309-58615-1, 408 pages, 6 x 9, (1996) This PDF is available from the National Academies Press at: http://www.nap.edu/catalog/2305.html Visit the National Academies Press online, the authoritative source for all books from the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council: ! Download hundreds of free books in PDF ! Read thousands of books online for free ! Explore our innovative research tools – try the “Research Dashboard” now! ! Sign up to be notified when new books are published ! Purchase printed books and selected PDF files Thank you for downloading this PDF. If you have comments, questions or just want more information about the books published by the National Academies Press, you may contact our customer service department toll- free at 888-624-8373, visit us online, or send an email to [email protected]. This book plus thousands more are available at http://www.nap.edu. Copyright © National Academy of Sciences. All rights reserved. Unless otherwise indicated, all materials in this PDF File are copyrighted by the National Academy of Sciences. Distribution, posting, or copying is strictly prohibited without written permission of the National Academies Press. Request reprint permission for this book. Lost Crops of Africa: Volume I: Grains http://www.nap.edu/catalog/2305.html i !"#$!"#$%%%&'"(#&'"(#&'"(#%%%")")")%%%*)'+,-*)'+,- volume I Grains 7(5(26.&868/7&"##9?&2#%&-7#;&%'/(5(26. -

Biocontrol of Sporobolus Grasses
Biocontrol of Sporobolus Grasses African survey for weedy sporobolus biocontrol agents Project number NBP.304 Report prepared for MLA by: Dr. W. A. Palmer Queensland Department of Natural Resources & Mines Meat & Livestock Australia Limited Locked Bag 991 North Sydney NSW 2059 ISBN 1 74036 508 9 March 2004 Natural Resources Biocontrol of Sporobolus Grasses TABLE OF CONTENTS Abstract ......................................................................................................................................................1 Executive Summary ...................................................................................................................................1 Introduction ................................................................................................................................................3 Methods .....................................................................................................................................................3 The Survey Area ........................................................................................................................................3 Identification of the Grasses .......................................................................................................................4 Collection of Specimens .............................................................................................................................5 Results .......................................................................................................................................................5 -
Variation in Plant Form in Recurrent Selection Populations of Kansas Rosinseed
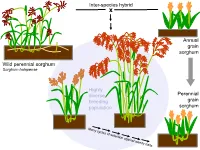
Inter-species hybrid x Annual grain sorghum Wild perennial sorghum Sorghum halapense Highly diverse Perennial breeding grain population sorghum 160 cm BULK06-10 perennial legumes 1. The Land Institute is domesticating Illinois bundleflower (IBF) – Adapted to Great Plains conditions – Nitrogen fixation and seed protein content similar to soybean Dehiscent Indehiscent Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 2. Existing forage legumes as nitrogen source for perennial cereals Mowed legume intercrop Multi-row mower tractor attachment Late spring: Mow between each row of cereal Early summer: Mowing triggers legume to drop fine roots; nitrogen from decomposing roots and leaves is taken up by the cereal crop 3. Existing perennial grain legumes – but usually grown as annuals • pigeonpea • lab-lab • runner bean • Lima bean Pigeon Pea •Deeply-rooted, perennial •4.92 million hectares worldwide (3.58 million in India alone) •Average yield 898 kg/ha ―The pigeonpea plants, especially of the perennial varieties, have a strong root system, which helps hold the soil on sloping hillsides. ― ―’Pigeonpea has been found to be very successful in covering the soil and reducing soil erosion," says Dr Zong Xuxiao, from the Chinese Academy of Agricultural Sciences at Beijing.’‖ http://www.cgiar.org/newsroom/releases/news.asp?idnews=536 -
Redalyc.Nutritive Value of Date Palm Leaves and Aristida Pungens
Tropical and Subtropical Agroecosystems E-ISSN: 1870-0462 [email protected] Universidad Autónoma de Yucatán México Arhab, Rabah; Macheboeuf, Didier; Doreau, Michel; Bousseboua, Hacéne Nutritive value of date palm leaves and Aristida pungens estimated by chemical, in vitro and in situ methods Tropical and Subtropical Agroecosystems, vol. 6, núm. 3, 2006, pp. 167-175 Universidad Autónoma de Yucatán Mérida, Yucatán, México Available in: http://www.redalyc.org/articulo.oa?id=93960306 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Tropical and Subtropical Agroecosystems 6 (2006): 167-175 SHORT NOTE [NOTA CORTA] NUTRITIVE VALUE OF DATE PALM LEAVES AND Aristida pungens Tropical and ESTIMATED BY CHEMICAL, IN VITRO AND IN SITU METHODS [VALOR NUTRITIVO DE LAS HOJAS DE PALMERA Y DE Aristida pungens Subtropical ESTIMADO POR METODOS QUÍMICOS, IN VITRO E IN SITU] Agroecosystems Rabah Arhab1,2*, Didier Macheboeuf1, Michel Doreau1, Hacène Bousseboua2 1Unité de Recherches sur les Herbivores, Institut National de la Recherche Agronomique, 63122 Saint-Genès Champanelle, France. 2Laboratoire de Génie Microbiologique et Applications, Département des Sciences de la Nature, Faculté des Sciences, Université Mentouri, 25000 Constantine, Algeria, Tel.: 00 33 4 73 62 47 29; fax: 00 33 4 73 62 42 73. E-mail: [email protected] *Corresponding author SUMMARY RESUMEN This study was carried out to determine the nutritive Se determinó el valor nutricio de dos forrajes de zonas value of two forages from the South-East of Algeria. -
Safe and Non-Toxic Hydroxyalkylation of Xylan Using Propylene Carbonate European Polymer Journal, 2016, 77, 88-97
Modifikation von Xylanen mit cyclischen organischen Carbonaten (Modification of xylans by cyclic organic carbonates) DISSERTATION zur Erlangung der Würde des Doktor der Naturwissenschaften (Dr. rer. nat.) Der Fakultät für Mathematik, Informatik und Naturwissenschaften Fachbereich Biologie Der Universität Hamburg Vorgelegt von YOUSSEF AKIL aus Stade Hamburg 2019 I II Tag der Disputation: 05. Juli 2019 Gutachter: Prof. Dr. Bodo Saake Universität Hamburg Fachbereich Biologie Institut für Holzwissenschaften Prof. Dr. Sascha Rohn Universität Hamburg Fachbereich Chemie Institut für Lebensmittelchemie III Danksagung Zu allererst gilt mein Dank Prof. Dr. Bodo Saake für die Überlassung dieses spannenden und herausfordernden Themas und die Aufnahme in seine Arbeitsgruppe. Ich bin dankbar für die gewährten Freiheiten und Hilfestellungen, welche signifikant zum Erfolg dieser Arbeit beigetragen haben. Ich bedanke mich auch bei Prof. Dr. Sascha Rohn für die freundliche Übernahme des Zweitgutachtens. Ebenfalls gilt mein Dank Prof. Dr. Tatiana Budtova und Dr. Romain Castellani, welche mir einen erfolgreichen und sehr lehrreichen Aufenthalt in Sophia-Antipolis ermöglicht haben. Einen besonderen Dank möchte ich Dr. Ralph Lehnen aussprechen, welcher immer ein offenes Ohr für mich hatte und mir bei einer Vielzahl an Fragestellungen mit Rat und Tat zur Seite stand. Gleiches gilt für Dr. Othar Kordsachia, welcher für mich ebenfalls ein zuverlässiger Ansprechpartner für verschiedenste Anliegen war. Ich bedanke mich besonders bei Inge Stichweh für die dauerhafte Hilfe bei der Chemikalienbeschaffung und –bereitstellung. Ebenfalls gilt ein besonderer Dank Bernhard Ziegler für viele NMR-Messungen und Hilfestellungen rund um das Gerät. Ein großer Dank gilt allen Kollegen und Kolleginnen, welche die Arbeit in den letzten 4 Jahren einladend und abwechslungsreich gestaltet haben. -

Comparing Chemical Composition and Digestibility of Pedicels and Palm Leaves As a Source for Livestock Feeding by in Vitro and in Situ Techniques
Journal of Rangeland Science, 2019, Vol. 9, No. 4 Boufennara et al.,/ 378 Archive of SID Contents available at ISC and SID Journal homepage: www.rangeland.ir Research and Full Length Article: Comparing Chemical Composition and Digestibility of Pedicels and Palm Leaves as a Source for Livestock Feeding by in vitro and in situ Techniques Souhil BoufennaraAC*, Lyas BouazzaA, Samir MedjekalB, Khaled RahalA, Ivan MateosC, Maria Jose RanillaC, SecundinoLopezC A Department of Cellular and Molecular Biology, Faculty of Nature Sciences, University Abbes Laghrour of Khenchela, 40000. Khenchela, Algeria *(Corresponding author), Email: [email protected] B Department of Microbiology and Biochemistry, Faculty of Sciences, Med Boudiaf University of M’sila, Bp166, 28000, M’sila, Algeria C Instituto de Ganadería de Montaña, CSIC-Universidad de León, Departamento de Producción Animal, Universidad de León, 24007 León, Spain Received on: 28/11/2018 Accepted on: 18/03/2019 Abstract. The aim of this study was to determine the chemical composition and in vitro digestibility, and to estimate the in vitro fermentation parameters of palm leaves and pedicels from Ghars palm variety. A rumen in situ technique was also used to examine In vitro Digestible Dry Matter (IDDM), In vitro Digestible Crude Protein (IDCP), and In vitro Digestible Neutral Fiber (IDNF), and rate and extent of gas production. Vetch-oat hay was taken as a reference feed material. The samples were collected in Tolga district (southeast Algeria). TheCrude Protein (CP) content of the plant species was low for pedicels and palm leaves (33 and 60 g kg−1 DM, respectively). The highest content of total extractable phenols, tannins and condensed tannins was observed in palm leaves whereas pedicels showed lower concentrations. -

Genetic Considerations in Ecosystem Restoration Using Native Tree Species the State of the World’S Forest Genetic Resources Thematic Study
GENETIC CONSIDERATIONS IN ECOSYSTEM RESTORATION USING NATIVE TREE SPECIES USING NATIVE IN ECOSYSTEM RESTORATION GENETIC CONSIDERATIONS THE STATE OF THE WORLD’S FOREST GENETIC RESOURCES THEMATIC STUDY There is renewed interest in the use of native tree species in ecosystem restoration for their biodiversity benefits. Growing native tree species in production systems THE STATE OF THE WORLD’S FOREST GENETIC RESOURCES – THE STATE (e.g. plantation forests and subsistence agriculture) can also ensure landscape functionality and support for human livelihoods. Achieving full benefits, however, requires consideration of genetic aspects that are often neglected, such as suitability of germplasm to the site, quality and quantity of the genetic pool used and regeneration potential. Understanding the extent and nature of gene flow across fragmented agro-ecosystems is also crucial to successful ecosystem restoration. This study, prepared within the ambit of The State of the World’s Forest Genetic Resources, reviews the role of genetic considerations in a wide range of ecosystem restoration activities involving trees. It evaluates how different approaches take, or could take, genetic aspects into account, thereby leading to the identification and selection of the most appropriate methods. The publication includes a review and syntheses of experience and results; an analysis of successes and failures in various systems; and definitions of best practices including genetic aspects. It also identifies knowledge gaps and needs for further research and development -

Appendix List of Higher Plant Names with Authors, Synonyms, and Family Name
Appendix List of Higher Plant Names with Authors, Synonyms, and Family Name Plant names with = sign before the name are synonyms or names that were commonly used for other plants in the past even if not strict taxonomic synonyms. Abronia maritima S. Watson = Aristida sabulicola (see Stipagrostis) [Nyctaginaceae] = Aristida scoparia (see Stipagrostis) Abronia umbellata Lam. [Nyctaginaceae] Artemisia monosperma Delile [Asteraceae] Abronia villosa S. Watson [Nyctaginaceae] Artemisia tridentata Nutt. [Asteraceae] Acacia albida Delile [Mimosaceae] = Arthrocnemum glaucum (see A. Achillea millefolium 1. [Asteraceae] macrostachyum) Achnatherum hymenoides (Roemer et Arthrocnemum macrostachyum (Moric.) Schultes) Barkworth [Poaceae] Moris Delponte [Chenopodiaceae] Adonis dentata Delile [Ranunculaceae] Arundo donax 1. [Poaceae] Aeluropus lagopoides (1.) Twaites [Poaceae] = Asphodelus microcarpus Salzm. et Vivo Agathophora alopecuroides (Delile) Bunge (see A. ram os us) [Chenopodiaceae] Asphodelus ramosus 1. [Liliaceae] Agropyron dasystachyum (Hooker) Scrib. = Asthenatherum forskalii (Vahl) Nevski [Poaceae] (See Centropodia) = Agropyron junceum (1.) Beauv. (see Astragalus annularis Forssk. [Fabaceae] Elymus farctus) Astragalus camelorum Barbey [Fabaceae] Ambrosia chamessonis (Less.) E. Greene Astragalus caprin us 1. [Fabaceae] [Asteraceae] A trip lex polycarpa (Torrey) S. Watson Ambrosia dumosa (Gray) Payne [Asteraceae] [Chenopodiaceae] Ammochloa palaestina Boiss. [Poaceae] A vena wiestii Steudel [Poaceae] Ammophila arenaria (1.) Link [Poaceae] Ammophila -

5 Wild-Grass Seed Harvesting in the Sahara and Sub-Sahara of Africa JACK R
5 Wild-grass seed harvesting in the Sahara and Sub-Sahara of Africa JACK R. HARLAN Introduction Over 60 species of grasses have been harvested for their grains in Africa. Most of these are famine or scarcity foods or are harvested casually and opportunistically. Several species, however, have provided food on a massive scale and have been staples for a number of tribes. These include: Aristida pungens, a staple of the northern Tuareg; Panicum turgidum, basic in central Sahara; Cenchrus biflorus, a major food of the Sahel; krebs, a complex of a dozen or more species including Panicum laetum, several species of Eragrostis, Dactyloctenium, Brachiaria, etc., providing staples from Bornu to Kordofan; Oryza barthii, widely harvested from swamps in the savanna, but a staple from Bornu to Kordofan; Paspalum scrobiculatum, a companion weed-crop of rice in the forest zone of West Africa; and Echinochloa stagnina, a sugar crop as well as a grain crop in the central Niger delta and around Lake Chad. Methods of harvest include: beating into a container, rubbing with the hands into a basket, cutting with a knife or sickle and threshing with a stick; using a swinging basket or calabash; sweeping or raking off the sand; tying up clumps with string before harvest. The grains are consumed in many forms: pounded and eaten raw; as dilute flour or semoula in a drink; gruel; porridge; flat bread; pastry; couscous; and beer. Bran may be removed in the mortar and used separately. Some of these practices may have started in the Palaeolithic, and they all have relevance to the processes of plant domestication and the beginnings of agriculture.