27-2-Erraigkarl HASS at TORNEYS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2016/022464 Al 11 February 2016 (11.02.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/022464 Al 11 February 2016 (11.02.2016) P O P C T (51) International Patent Classification GELMAN, Leonid; 260 E. Grand Avenue, 2nd Floor, A61K 31/7068 (2006.01) A61K 31/519 (2006.01) South San Francisco, CA 94080 (US). SMITH, David, A61K 31/4184 (2006.01) A61K 31/166 (2006.01) Bernard; 260 E. Grand Avenue, 2nd Floor, South San A61K 31/675 (2006.01) A61K 31/41 (2006.01) Francisco, CA 94080 (US). WANG, Guangyi; 260 E. A61K 31/513 (2006.01) A61K 38/21 (2006.01) Grand Avenue, 2nd Floor, South San Francisco, CA 94080 A61K 31/506 (2006.01) A61K 38/16 (2006.01) (US). A61K 31/437 (2006.01) A61K 39/155 (2006.01) (74) Agent: MILLER, Kimberly, J.; Knobbe Martens Olson A61K 31/4709 (2006.01) A61K 39/42 (2006.01) & Bear, LLP, 2040 Main Street, 14th Floor, Irvine, CA A61K 31/4188 (2006.01) A61P 31/14 (2006.01) 92614 (US). A61K 31/517 (2006.01) A61P 11/00 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: PCT/US20 15/043402 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) International Filing Date: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 3 August 2015 (03.08.2015) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, English (25) Filing Language: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (26) Publication Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (30) Priority Data: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/033,55 1 5 August 2014 (05.08.2014) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

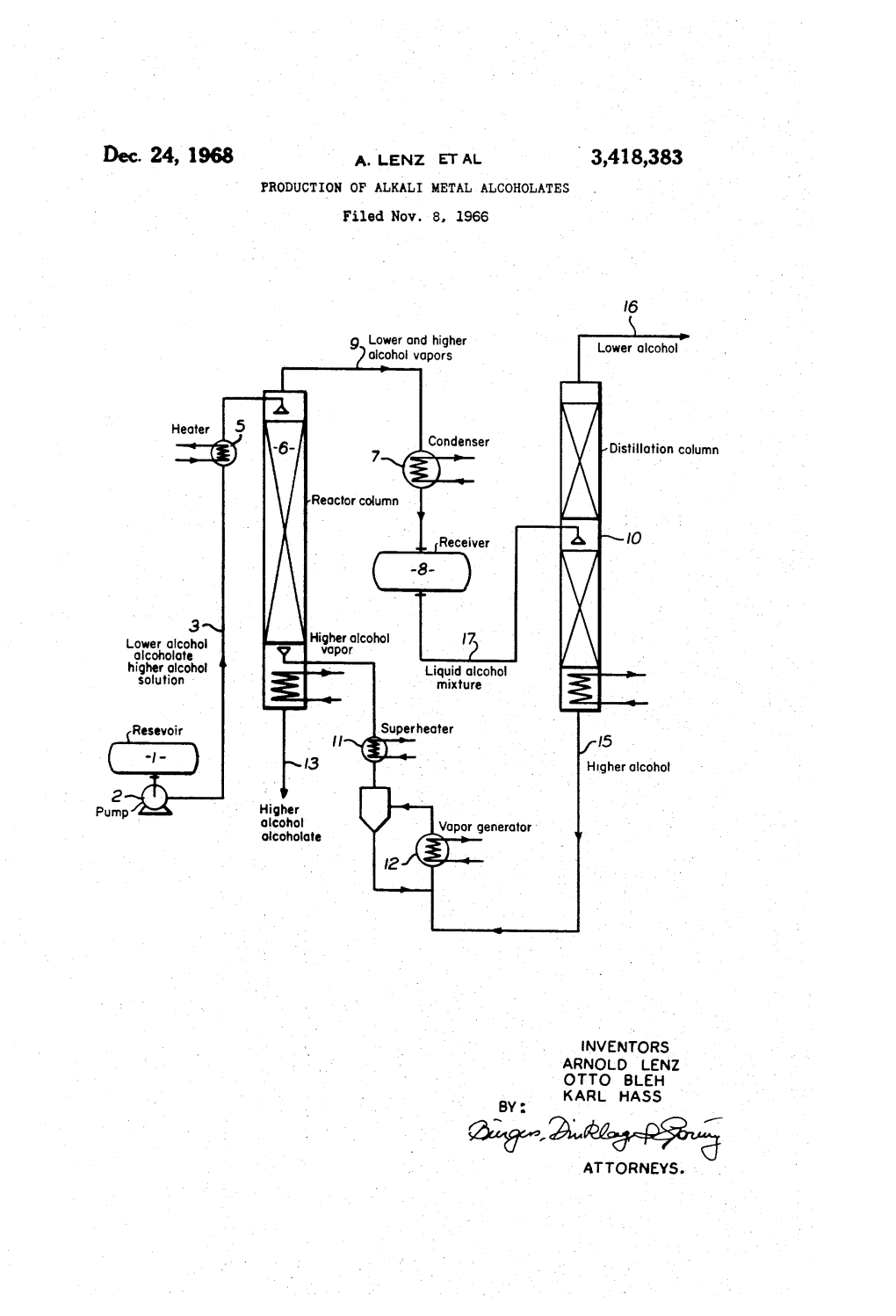
United States Patent Office Patented Jan
3,164,611 United States Patent Office Patented Jan. 5, 1965 1. 2 reaction, care must be taken in applying this method 3,164,611 to the oxidation of heat sensitive compounds. OXDATION OF PRIMARY AND SECONDARY AL COHOLS TO THE CORRESPONDING CARBONY These organic base-chromium trioxide complexes are CSCMPOUNDS (USNG A TERTARY AMENE particularly useful oxidizing agents for effecting the oxida (CERORySSJR, TROXDE COMPLEX tion of alcohols having at least one hydrogen atom at Lewis H. Sarett, Friscetos, N.J., assigaor to Merck & Co., tached to the carbon atom bearing the hydroxyl sub Inc., Rahway, N.J., a corporatioia of New Jersey Stituent, i.e., primary and secondary alcohols, to the No Drawing. Fied any 26, 1956, Ser. No. 686,463 corresponding carbonyl compounds. Thus, primary al 13 Caias. (C. 260-349.9) cohols are oxidized to aldehydes, and secondary alcohols IO are converted to ketones. This invention relates to a novel process for the oxida This method of oxidizing alcohols to the corresponding tion of chemical compounds, and more particularly to carbonyl compounds is generally applicable to all pri an improved method for the oxidation of primary and mary and secondary alcohols. Examples of such al Secondary alcohols to the corresponding carbonyl com cohols that might be mentioned are aliphatic alcohols pounds. 5 such as alkanals, alkenols, alkinois, polyhydric alkanols, This application is a continuation-in-part application polyhydric alkenols and polyhydric alkinols; aralkyl al of my application Serial No. 263,016, filed December cohols; aralkenyl alcohols; aralkinyl alcohols; alicyclic 22, 1951, now abandoned, and my copending application alcohols such as cycloalkyl, cycloalkenyl, cycloalkinyl, Serial No. -

A Review on Green Liquid Fuels for the Transportation Sector: a Prospect of Microbial Solutions to Climate Change
Biofuel Research Journal 23 (2019) 995-1024 Journal homepage: www.biofueljournal.com Review Paper A review on green liquid fuels for the transportation sector: a prospect of microbial solutions to climate change Hamed Kazemi Shariat Panahi1,2, Mona Dehhaghi1,2, James E. Kinder 3, Thaddeus Chukwuemeka Ezeji3,* 1 Faculty of Medicine and Health Sciences, Macquarie University, NSW, Australia. 2Department of Microbial Biotechnology, School of Biology and Centre of Excellence in Phylogeny of Living Organisms, College of Science, University of Tehran, Tehran, Iran. 3Department of Animal Sciences, Ohio State Agricultural Research and Development Center (OARDC), The Ohio State University, Wooster, USA. HIGHLIGHTS GRAPHICAL ABSTRACT Microbial-based biofuel as a promising waste-to- energy technology has been scrutinized. Microbial production of bio-jet fuel is possible through DSHC, AtJ, and GtL. Future application of ammonia as bio-fuel requires special design of ICE. Cons and pros of microbial liquid fuels over gasoline have been outlined. Conversion of microbial liquid fuel into fuel derivatives has been discussed. ARTICLE INFO ABSTRACT Article history: Environmental deterioration, global climate change, and consequent increases in pollution-related health problems among Received 12 July 2019 populations have been attributed to growing consumption of fossil fuels in particular by the transportation sector. Hence, Received in revised form 18 August 2019 replacing these energy carriers, also known as major contributors of greenhouse gas emissions, with biofuels have been regarded Accepted 20 August 2019 as a solution to mitigate the above-mentioned challenges. On the other hand, efforts have been put into limiting the utilization Available online 1 September 2019 of edible feedstocks for biofuels production, i.e., first generation biofuels, by promoting higher generations of these eco-friendly alternatives. -

Alcohol Oxidase from Hansenula Polymorpha
Alcohol Oxidase from Hansenula polymorpha Catalog Number A0438 Storage Temperature –20 C CAS RN 9073-63-6 Molecular mass:6 670 kDa (octomer, gel filtration) EC 1.1.3.13 Synonym: Alcohol:oxygen oxidoreductase Alcohol oxidase is a homooctomeric flavoprotein with eight equal subunits of 83 kDa; each of which contains Product Description a flavin adenine dinucleotide (FAD) molecule.6 Alcohol oxidase catalyzes the oxidation of short-chain, primary, aliphatic alcohols to the respective aldehydes. Cofactor:4 FAD, one molecule/subunit 7 RCH2OH + O2 RCHO + H2O2 Isoelectric point: 6.1 The enzyme has the highest affinity for methanol with pH Range:8 6.7–9.8 the affinity decreasing with increasing chain length of the alkyl (R) group. pH Optimum:8 8.5 5 Alcohol oxidase plays a major role in the metabolism of Ki (mM): methanol resulting in the formation of formaldehyde and methanol 6,500 has been detected in several genera of yeasts, such as Candida, Pichia, and Hansenula, that utilize methanol Inhibitors:3,8 as a sole carbon and energy source.1,2 1,4-butynediol (irreversible) 4-hydroxy-2- butynal Primarily localized in the peroxisome, alcohol oxidase cyclopropanol has also been found in the cytoplasm. Monomers are cyclopropanone (suicide substrate) synthesized in the cytosol and assembled into octomers formaldehyde, H2O2 (5–10 mM) in the peroxisome. Octomerization is thought to be hydroxylamine, KBr, KCN chaparone mediated.3 Alcohol oxidase is of interest for sodium azide, urea the study of protein translocation into peroxisomes.4 This product is purified from Hansenula polymorpha 4,5 KM (mM): and is supplied as an orange vacuum-dried powder. -

Journal of International Scientific Publications: Materials, Methods & Technologies Volume 6
Journal of International Scientific Publications: Materials, Methods & Technologies, Volume 6, Part 2 ISSN 1313-2539, Published at: http://www.science-journals.eu FERMENTATIVE BIOFUELS PRODUCTION Flora V. Tsvetanova and Kaloyan K. Petrov* Institute of Chemical Engineering, Bulgarian Academy of Sciences, Acad. G. Bonchev str., bl. 103, Sofia, Bulgaria, *E-mail: kaloian04@vahoo .com A b stract The limited reserves and increasing prices o f fossil carbohydrates, as well as the global warming due to their utilization, impose the finding o f renewable energy sources. Because o f this, since decades an increasing interest in production o f alcohols, which can be used as a fuel additives or fuels for direct replacement in gasoline engines, is observed. Alcohols can be obtained chemically or as products o f microbial metabolism o f different species in fermentation of sugars or starchy materials. In the present review are summarized different fermentative pathways for production of all alcohols, which are or could be used as biofuels. The focus o f the paper is on production limitations, strains development and economical perspectives. Key words:fermentation, biofuel, alcohols 1. IN T R O D U C T IO N The increasing energy demand and running low stocks of fossil fuels in last decades shift the attention to alcohols production in respect of their fuel properties. Alcohols can be produced from renewable sources and reply both to energy crisis and environmental problems. A number of alcohols are candidates to replace the existed fuels, but to date only ethanol and methanol fuels are on race. Having in mind that methanol fuel is produced mainly from coal or natural gas, it remains that only ethanol fuel is received by fermentation. -

European Patent Office © Publication Number: 0 026 547 A1 Office Europeen Des Brevets
Europaisch.es Patentamt European Patent Office © Publication number: 0 026 547 A1 Office europeen des brevets © EUROPEAN PATENT APPLICATION © Application number: 80200911.8 © Int. CI.3: C 07 F 3/00 C 07 C 31/30, C 07 C 29/68 © Date of filing: 26.09.80 //C08G65/28, C07C41/03, B01J31/02 © Priority: 27.09.79 US 79497 © Applicant: UNION CARBIDE CORPORATION 270, Park Avenue New York, N.Y. 10017(US) © Date of publication of application: 08.04.81 Bulletin 81/14 @ Inventor: McCain, James Herndon 1987 Parkwood Road © Designated Contracting States: Charleston West Virginia 25314(US) BE DE FR GB IT NL SE © Inventor: Foster, Donald Joseph 603 39th Street, S.E. Charleston West Virginia 25304(US) © Representative: Urbanus, Henricus Maria, Ir. et al, c/o Vereenigde Octrooibureaux Nieuwe Parklaan 107 NL-2587 BP 's-Gravenhage(NL) © Process for the preparation of basic salts of alkaline earth metals and basic salts obtained by this process. (57) A process is provided for the preparation of soluble basic salts of alkaline earth metals that are catalytically active in the oxyalkylation reaction of alcohols, polyols and phenols which comprises reacting an alkaline earth metal material selected from the group consisting of calcium, strontium, and barium and mixtures of the same with a lower monohydric alcohol having 1 to 7 carbon atoms at a temperature at which the reaction proceeds to form a lower alcohol metal alkoxide, mixing a polyol or a higher monohydric alcohol having at least 4 carbon atoms with the lower alcohol-alkaline earth metal alkoxide reaction product and removing the lower alcohol therefrom. -

Ring Expansion of Cyclobutylmethylcarbenium Ions to Cyclopentane Or Cyclopentene Derivatives and Metal-Promoted Analogous Rearrangements
Ring expansion of cyclobutylmethylcarbenium ions to cyclopentane or cyclopentene derivatives and metal-promoted analogous rearrangements Erika Leemans, Matthias D‟hooghe, Norbert De Kimpe* Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium *[email protected] Table of Contents 1 Introduction ........................................................................................................................ 2 2 Ring expansion of cyclobutylmethylcarbenium ions through activation of a carbon- carbon double bond .................................................................................................................... 5 2.1 Acid-promoted activation of alkenylcyclobutanes ...................................................... 7 2.1.1 Pinene rearrangement ........................................................................................... 7 2.1.2 Ring expansion of vinylcyclobutanes (different from pinene) ............................. 9 2.1.3 Semipinacol rearrangement of 1-vinylcyclobutanols ......................................... 17 2.2 Halogen/selenium cation-promoted activation .......................................................... 23 2.3 Metal-promoted activation ........................................................................................ 27 2.3.1 Mercury-promoted activation ............................................................................. 27 2.3.2 Palladium-promoted activation ......................................................................... -

Environmental Health Criteria 167 ACETALDEHYDE
Environmental Health Criteria 167 ACETALDEHYDE Please note that the layout and pagination of this web version are not identical with the printed version. Acetaldehyde (EHC 167, 1995) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 167 ACETALDEHYDE This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. First draft prepared by Mrs. J. de Fouw, National Institute of Public Health and Enviromental Protection, Bilthoven, Netherlands Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Organization Geneva, 1995 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. WHO Library Cataloguing in Publication Data Acetaldehyde. (Environmental health criteria ; 167) 1.Acetadehyde - adverse effects 2.Enviromental exposure I.Series Page 1 of 86 Acetaldehyde (EHC 167, 1995) ISBN 92 4 157167 5 (NLM Classification: QU 99) ISSN 0250-863X The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. -
Lipophilicity Trends Upon Fluorination of Isopropyl, Cyclopropyl and 3-Oxetanyl Groups
Lipophilicity trends upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl groups Benjamin Jeffries1, Zhong Wang1, Robert I. Troup1, Anaïs Goupille2, Jean-Yves Le Questel2, Charlene Fallan3, James S. Scott3, Elisabetta Chiarparin3, Jérôme Graton2 and Bruno Linclau*1 Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2020, 16, 2141–2150. 1School of Chemistry, University of Southampton, Highfield, https://doi.org/10.3762/bjoc.16.182 Southampton SO17 1BJ, UK, 2Université de Nantes, CNRS, CEISAM UMR 6230, F-44000 Nantes, France and 3Medicinal Chemistry, Received: 19 July 2020 Oncology R&D, AstraZeneca, Cambridge CB4 0WG, UK Accepted: 20 August 2020 Published: 02 September 2020 Email: Bruno Linclau* - [email protected] This article is part of the thematic issue "Organo-fluorine chemistry V". * Corresponding author Guest Editor: D. O'Hagan Keywords: © 2020 Jeffries et al.; licensee Beilstein-Institut. aliphatic fluorination; cyclopropane; isopropyl; isostere; lipophilicity; License and terms: see end of document. oxetane Abstract A systematic comparison of lipophilicity modulations upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl substituents, at a single carbon atom, is provided using directly comparable, and easily accessible model compounds. In addition, comparison with relevant linear chain derivatives is provided, as well as lipophilicity changes occurring upon chain extension of acyclic precursors to give cyclopropyl containing compounds. For the compounds investigated, fluorination of the isopropyl substituent led to larger lipophilicity modulation compared to fluorination of the cyclopropyl substituent. Introduction The introduction of small alkyl groups onto bioactive com- example, an isopropyl and a trifluoromethyl group have very pounds as space filling groups is a common strategy in the drug similar volumes, but a very different shape [6]. -
Module IV: Chemistry of Functional Groups – I (9 Hrs) Halogen Compounds: Preparation of Alkyl Halides from Alkanes and Alkenes
Module IV: Chemistry of Functional Groups – I (9 hrs) Halogen Compounds: Preparation of alkyl halides from alkanes and alkenes - Wurtz reaction and 1 2 Fittig’s reaction - Mechanism of SN and SN reactions of alkyl halides – Effect of substrate and stereochemistry. Alcohols: Preparation from Grignard reagent - Preparation of ethanol from molasses - Wash, rectified spirit, absolute alcohol, denatured spirit, proof spirit and power alcohol (mention only) – Comparison of acidity of ethanol, isopropyl alcohol and tert-butyl alcohol - Haloform reaction and iodoform test - Luca’s test - Chemistry of methanol poisoning – Harmful effects of ethanol in the human body. Phenols: Preparation from chlorobenzene – Comparison of acidity of phenol, p-nitrophenol and pmethoxyphenol – Preparation and uses of phenolphthalein. Ethers: Preparation by Williamson’s synthesis – Acidic cleavage - Crown ethers (mention only). Preparation of alkyl halides from alkanes Alkanes (the most basic of all organic compounds) undergo very few reactions. One of these reactions is halogenation, or the substitution of single hydrogen on the alkane for a single halogen to form a haloalkane. When methane (CH4) and chlorine (Cl2) are mixed together in the presence of ultra violet irradiation, product is formed, chloromethane (CH3Cl). The reaction proceeds through the radical chain mechanism. The radical chain mechanism is characterized by three steps: initiation, propagation and termination. Initiation requires an input of energy but after that the reaction is self-sustaining. The first propagation step uses up one of the products from initiation, and the second propagation step makes another one, thus the cycle can continue until indefinitely. Step 1: Initiation: Initiation breaks the bond between the chlorine molecule (Cl2). -

Untargeted Metabolomics-Like Screening Approach for Chemical
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2018 Electronic Supplementary Information Untargeted metabolomics-like screening approach for chemical characterization and differentiation of canopic jar and mummy samples from Ancient Egypt using GC-high resolution MS Lana Brockbals 1, Michael Habicht 2, Irka Hajdas 3, Francesco M. Galassi 2, Frank J. Rühli 2° and Thomas Kraemer 1°* 1 Department of Forensic Pharmacology and Toxicology, Zurich Institute of Forensic Medicine, University of Zurich, Zurich, Switzerland 2 Institute of Evolutionary Medicine, University of Zurich, Zurich, Switzerland 3 Institute of Ion Beam Physics, ETH Zurich, Zurich, Switzerland ° shared last authorship Table ESI 1: List of positive identified hits based on screening against the NIST 14 spectral library; name of substance (given as corresponding methoximation (MOX) and/or trimethylsilyl (TMS) derivative where applicable) with corresponding positive sample(s) indexed as in table 1 of the original paper; substances sorted according to retention time. Name of substance (given as MOX/ TMS derivative where Positive samples [index] applicable) 3-Methylbutanoic acid TMS derivative 7, 9, 18, 29 Acetamide, 2,2,2-trifluoro-N-methyl- 16, 17 acetamide, 2,2,2-trifluoro-N-(2-hydroxyethyl)- 26, 29, 30, 31 Urea 2TMS derivative 5 Aniline 12, 13, 14 Acetamide, N-butyl-2,2,2-trifluoro- 3, 4, 7, 12, 13, 14, 15 Urea 2TMS derivative 5 Bicyclo[3.2.0]hepta-2,6-diene 2, 13, 19, 26, 28 2(5H)-Furanone, 5,5-dimethyl- 7 Pentanoic acid, -
United States Patent 19 11) Patent Number: 4,644,007 York, Jr
United States Patent 19 11) Patent Number: 4,644,007 York, Jr. 45 Date of Patent: * Feb. 17, 1987 54) 3-CHLORO-4-(4,5-DIHYDRO-1H-MIDAZO 2832310 7/1980 Fed. Rep. of Germany. 2-YL)-AMINO-5-ALKYLBENZOIC ACIDS, 2905883 8/1980 Fed. Rep. of Germany . 2949287 11/1981 Fed. Rep. of Germany . ESTERS, SALTS, COMPOSITIONS AND 79.2696 6/1980 South Africa . METHODS 1180766 10/1967 United Kingdom. 75) Inventor: Billie M. York, Jr., Fort Worth, Tex. 1216945 12/1970 United Kingdom. 1279543 6/1972 United Kingdom. 73) Assignee: Alcon Laboratories, Inc., Fort 1279931 6/1972 United Kingdom. Worth, Tex. 15954.12 8/1981 United Kingdom . * Notice: The portion of the term of this patent OTHER PUBLICATIONS subsequent to May 14, 2002 has been disclaimed. DeJonge, Europ. J. of Pharm. 71 (1981) 411-420; “Dis crimination Between Peripheral and Central Alpha-A- 21) Appl. No.: 755,373 drenergic Effects Using Meta-Substituted Imidazo 22 Filed: Jul. 15, 1985 lines'. J. Pharmcol. Methods 6(2) 109-20 (1981)-Stahle et al. Related U.S. Application Data J. Labelled Compa. Radiopharm. 17(1), 35-41 (1980), 63. Continuation of Ser. No. 590,464, Mar. 16, 1984, aban Rouot et al. doned, which is a continuation-in-part of Ser. No. J. Med. Chem. 24, 502-507 (1981)-Pieter et al. 519,791, Aug. 3, 1983, Pat. No. 4,517,199, and Ser. No. Naunyn-Schmiedeberg's Pharmacol. 317(8), 1-12 520,071, Aug. 3, 1983, Pat. No. 4,515,800, which is a (1981), DeJonge et al. continuation-in-part of Ser.