The Observed Alteration in BCL2 Expression Following Lithium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anti-CA1 / Carbonic Anhydrase 1 Antibody (ARG65670)
Product datasheet [email protected] ARG65670 Package: 100 μl, 50 μl anti-CA1 / Carbonic Anhydrase 1 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes CA1 / Carbonic Anhydrase 1 Tested Reactivity Ms, Rat Tested Application WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name CA1 / Carbonic Anhydrase 1 Antigen Species Human Immunogen Full length fusion protein of Human CA1. Conjugation Un-conjugated Alternate Names Carbonic anhydrase I; EC 4.2.1.1; Carbonate dehydratase I; Carbonic anhydrase B; Car1; HEL-S-11; CA-I; Carbonic anhydrase 1; CAB Application Instructions Application table Application Dilution WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control WB: Mouse liver tissue Calculated Mw 29 kDa Properties Form Liquid Purification Affinity purification with immunogen. Buffer PBS (pH 7.3), 0.05% Sodium azide and 50% Glycerol Preservative 0.05% Sodium azide Stabilizer 50% Glycerol Concentration 0.9 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Gene Symbol CA1 Gene Full Name carbonic anhydrase I Background Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide. -

Neglected Functions of TFCP2/TFCP2L1/UBP1 Transcription Factors May Offer Valuable Insights Into Their Mechanisms of Action
International Journal of Molecular Sciences Review Neglected Functions of TFCP2/TFCP2L1/UBP1 Transcription Factors May Offer Valuable Insights into Their Mechanisms of Action Agnieszka Taracha, Grzegorz Kotarba and Tomasz Wilanowski * Laboratory of Signal Transduction, Nencki Institute of Experimental Biology of Polish Academy of Sciences, 3 Pasteur St., 02-093 Warsaw, Poland; [email protected] (A.T.); [email protected] (G.K.) * Correspondence: [email protected]; Tel.: +48-22-5892-311 Received: 21 August 2018; Accepted: 19 September 2018; Published: 20 September 2018 Abstract: In recent years, the TFCP2 (transcription factor cellular promoter 2)/TFCP2L1 (TFCP2- like 1)/UBP1 (upstream binding protein 1) subfamily of transcription factors has been attracting increasing attention in the scientific community. These factors are very important in cancer, Alzheimer’s disease, and other human conditions, and they can be attractive targets for drug development. However, the interpretation of experimental results is complicated, as in principle, any of these factors could substitute for the lack of another. Thus, studying their hitherto little known functions should enhance our understanding of mechanisms of their functioning, and analogous mechanisms might govern their functioning in medically relevant contexts. For example, there are numerous parallels between placental development and cancer growth; therefore, investigating the roles of TFCP2, TFCP2L1, and UBP1 in the placenta may help us better understand their functioning in cancer, as is evidenced by the studies of various other proteins and pathways. Our review article aims to call the attention of the scientific community to these neglected functions, and encourage further research in this field. -

Engineered Type 1 Regulatory T Cells Designed for Clinical Use Kill Primary
ARTICLE Acute Myeloid Leukemia Engineered type 1 regulatory T cells designed Ferrata Storti Foundation for clinical use kill primary pediatric acute myeloid leukemia cells Brandon Cieniewicz,1* Molly Javier Uyeda,1,2* Ping (Pauline) Chen,1 Ece Canan Sayitoglu,1 Jeffrey Mao-Hwa Liu,1 Grazia Andolfi,3 Katharine Greenthal,1 Alice Bertaina,1,4 Silvia Gregori,3 Rosa Bacchetta,1,4 Norman James Lacayo,1 Alma-Martina Cepika1,4# and Maria Grazia Roncarolo1,2,4# Haematologica 2021 Volume 106(10):2588-2597 1Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 2Stanford Institute for Stem Cell Biology and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 3San Raffaele Telethon Institute for Gene Therapy, Milan, Italy and 4Center for Definitive and Curative Medicine, Stanford School of Medicine, Stanford, CA, USA *BC and MJU contributed equally as co-first authors #AMC and MGR contributed equally as co-senior authors ABSTRACT ype 1 regulatory (Tr1) T cells induced by enforced expression of interleukin-10 (LV-10) are being developed as a novel treatment for Tchemotherapy-resistant myeloid leukemias. In vivo, LV-10 cells do not cause graft-versus-host disease while mediating graft-versus-leukemia effect against adult acute myeloid leukemia (AML). Since pediatric AML (pAML) and adult AML are different on a genetic and epigenetic level, we investigate herein whether LV-10 cells also efficiently kill pAML cells. We show that the majority of primary pAML are killed by LV-10 cells, with different levels of sensitivity to killing. Transcriptionally, pAML sensitive to LV-10 killing expressed a myeloid maturation signature. -

Expression of Carbonic Anhydrase II
Biochemical Genetics, VoL 33, Nos. 11/12, 1995 Expression of Carbonic Anhydrase II (CA II) Promoter-Reporter Fusion Genes in Multiple Tissues of Transgenic Mice Does Not Replicate Normal Patterns of Expression Indicating Complexity of CA II Regulation in Vivo Robert P. Erickson, 1,2,4 Judy Grimes, 1 Patrick J. Venta, 3 and Richard E. Tashian 3 Received 6 June 1995 Final5 Sept. 1995 Although the proximal, 5' 115 bp of the human carbonic anhydrase II (CA II) gene was sufficient for expression of a reporter gene in some transfected cell lines, we found previously that 1100 bp of this promoter (or 500 bp of the mouse CA II promoter) was not sufficient for expression in transgenic mice. We have now studied the expression of linked reporter genes in mice transgenic for either (1) l 1 kb of the human 5' promoter or (2) 8 kb of the human 5' promoter with mouse sequences from the first exon, part of the first intron (since a CpG island spans this region), and the 3' sequences of the gene. Expression was found in both cases, but the tissue specificity was not appropriate for CA II. Although there was a difference in the sensitivity of the assays used, the frst construct led to expression in many tissues, while the second construct was expressed only in spleen. These findings indicate considerable complexity of DNA control regions for in vivo CA II expression. KEY WORDS: transgenic mice; carbonic anhydrase; promoter analysis; transcription; DNA control regions. Angel Charity for Children-Wings for Genetic Research, Steele Memorial Children's Re- search Center, Department of Pediatrics, University of Arizona, Tucson, Arizona. -

Regulation and Roles of Carbonic Anhydrases IX and XII
HEINI KALLIO Regulation and Roles of Carbonic Anhydrases IX and XII ACADEMIC DISSERTATION To be presented, with the permission of the board of the Institute of Biomedical Technology of the University of Tampere, for public discussion in the Auditorium of Finn-Medi 5, Biokatu 12, Tampere, on December 2nd, 2011, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere, Institute of Biomedical Technology Tampere University Hospital Tampere Graduate Program in Biomedicine and Biotechnology (TGPBB) Finland Supervised by Reviewed by Professor Seppo Parkkila Docent Peppi Karppinen University of Tampere University of Oulu Finland Finland Professor Robert McKenna University of Florida USA Distribution Tel. +358 40 190 9800 Bookshop TAJU Fax +358 3 3551 7685 P.O. Box 617 [email protected] 33014 University of Tampere www.uta.fi/taju Finland http://granum.uta.fi Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1675 Acta Electronica Universitatis Tamperensis 1139 ISBN 978-951-44-8621-0 (print) ISBN 978-951-44-8622-7 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2011 There is a crack in everything, that’s how the light gets in. -Leonard Cohen 3 CONTENTS CONTENTS .......................................................................................................... 4 LIST OF ORIGINAL COMMUNICATIONS...................................................... 7 ABBREVIATIONS ............................................................................................. -

Transcriptome Analysis of Hippocampal Subfields Identifies
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.05.935759. this version posted February 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. It is made available under a CC-BY 4.0 International license. Manuscript submitted to Hippocampus Transcriptome analysis of hippocampal subfields identifies gene expression profiles associated with long-term active place avoidance memory Rayna M. Harris1†, Hsin-Yi Kao2‡, Juan Marcos Alarcón3, André A. Fenton2,3, Hans A. Hofmann1 *For correspondence: [email protected] (RMH) 1Deptartment Integrative Biology; Institute for Cell and Molecular Biology, The 2 Present address: †Department of University of Texas at Austin; Center for Neural Science; Neuroscience Institute at the Neurobiology, Physiology and New York University Langone Medical Center, New York University; 3Dept. of Pathology; Behavior, University of California, Davis; ‡Neurology Department, The Robert F. Furchgott Center for Neural and Behavioral Science, SUNY Downstate University of Michigan, Ann Arbor Health Sciences University Abstract The hippocampus plays a critical role in storing and retrieving spatial information. By targeting the dorsal hippocampus and manipulating specific "candidate" molecules using pharmacological and genetic manipulations, we have previously discovered that long-term active place avoidance memory requires transient activation of particular molecules in dorsal hippocampus. These molecules include amongst others, the persistent kinases Ca-calmodulin kinase II (CaMKII) and the atypical protein kinase C isoform PKC / for acquisition of the conditioned behavior, whereas persistent activation of the other atypical PKC, protein kinase M zeta (PKM ) is necessary for maintaining the memory for at least a month. It nonetheless remains unclear what other molecules and their interactions maintain active place avoidance long-term memory, and the candidate molecule approach is both impractical and inadequate to identify new candidates since there are so many to survey. -

Investigating the Effects of Human Carbonic Anhydrase 1 Expression
Investigating the effects of human Carbonic Anhydrase 1 expression in mammalian cells Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Xiaochen Liu, BSc, MSc January 2016 ABSTRACT Amyotrophic Lateral Sclerosis (ALS) is one of the most common motor neuron diseases with a crude annual incidence rate of ~2 cases per 100,000 in European countries, Japan, United States and Canada. The role of Carbonic Anhydrase 1 (CA1) in ALS pathogenesis is completely unknown. Previous unpublished results from Dr. Jian Liu have shown in the spinal cords of patients with sporadic amyotrophic lateral sclerosis (SALS) there is a significant increased expression of CA1 proteins. The purpose of this study is to examine the effect of CA1 expression in mammalian cells, specifically, whether CA1 expression will affect cellular viability and induce apoptosis. To further understand whether such effect is dependent upon CA1 enzymatic activity, three CA1 mutants (Thr199Val, Glu106Ile and Glu106Gln) were generated using two- step PCR mutagenesis. Also, a fluorescence-based assay using the pH-sensitive fluorophore Pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid) to measure the anhydrase activity was developed. The assay has been able to circumvent the requirement of the specialized equipment by utilizing a sensitive and fast microplate reader and demonstrated that three - mutants are enzymatically inactive under the physiologically relevant HCO3 dehydration reaction which has not been tested before by others. The data show that transient expression of CA1 in Human Embryonic Kidney 293 (HEK293), African Green Monkey Kidney Fibroblast (COS7) and Human Breast Adenocarcinoma (MCF7) cell lines did not induce significant changes to the cell viability at 36hrs using the Water Soluble Tetrazolium-8 (WST8) assay. -

A Dissertation Entitled the Regulatory Role of Mixed Lineage Kinase 4
A Dissertation entitled The Regulatory Role of Mixed Lineage Kinase 4 Beta in MAPK Signaling and Ovarian Cancer Cell Invasion by Widian F. Abi Saab Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology _________________________________________ Dr. Deborah Chadee, Committee Chair _________________________________________ Dr. Douglas Leaman, Committee Member _________________________________________ Dr. Fan Dong, Committee Member _________________________________________ Dr. John Bellizzi, Committee Member _________________________________________ Dr. Max Funk, Committee Member _________________________________________ Dr. Robert Steven, Committee Member _________________________________________ Dr. William Taylor, Committee Member _________________________________________ Dr. Patricia R. Komuniecki, Dean College of Graduate Studies The University of Toledo May 2013 Copyright 2013, Widian Fouad Abi Saab This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of The Regulatory Role of Mixed Lineage Kinase 4 Beta in MAPK Signaling and Ovarian Cancer Cell Invasion by Widian F. Abi Saab Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology The University of Toledo May 2013 Mixed lineage kinase 4 (MLK4) is a member of the MLK family of mitogen- activated protein kinase kinase kinases (MAP3Ks). As components of a three-tiered signaling cascade, MAP3Ks promote activation of mitogen-activated protein kinase (MAPK), which in turn regulates different cellular processes including proliferation and invasion. Here, we show that the beta form of MLK4 (MLK4β), unlike its close relative, MLK3, and other known MAP3Ks, negatively regulates the activities of the MAPKs, p38, ERK and JNK, even in response to stimuli such as sorbitol or TNFα. -

Glucose Deficiency Elevates Acid-Sensing Ion Channel 2A
www.nature.com/scientificreports OPEN Glucose Deficiency Elevates Acid- Sensing Ion Channel 2a Expression and Increases Seizure Susceptibility Received: 20 September 2016 Accepted: 24 May 2017 in Temporal Lobe Epilepsy Published: xx xx xxxx Haitao Zhang, Guodong Gao, Yu Zhang, Yang Sun, Huanfa Li, Shan Dong, Wei Ma, Bei Liu, Weiwen Wang, Hao Wu & Hua Zhang Brain hypometabolism is a common epilepsy-related finding in both patients and animal models. Fluorodeoxyglucose positron emission tomography studies have shown that recurrent seizures lead to reduced glucose metabolism in certain brain regions, but no studies have definitively determined whether this induces epileptogenesis. There is evidence that acid-sensing ion channel 2a (ASIC2a) affects epilepsy susceptibility. Transcription factor CP2 (TFCP2) regulates ASIC2a expression. We report that suppressed TFCP2 expression and elevated ASIC2a expression were associated with glucose hypometabolism in the hippocampi of humans with epilepsy and of rat epilepsy model brains. In cultured PC12 cells, we determined that glucose deficiency led to TFCP2 downregulating ASIC2a. Moreover, electrophysiological recordings from cultured rat hippocampal slices showed that ASIC2a overexpression resulted in more action potentials in CA1 pyramidal neurons and increased seizure susceptibility. Our findings suggest that hippocampal glucose hypometabolism elevates ASIC2a expression by suppressing TFCP2 expression, which further enhances the intrinsic excitability of CA1 pyramidal neurons and increases seizure susceptibility in patients with temporal lobe epilepsy. Temporal lobe epilepsy (TLE) is the most common form of drug-resistant epilepsy and is characterised by recurrent and unprovoked seizures1. It is diagnosed from medical history, blood tests, and brain structural and functional imaging techniques including electroencephalography (EEG), computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI). -

Tip60 Might Be a Candidate for the Acetylation of Hepatic Carbonic Anhydrase I and III in Mice
Tip60 Might Be a Candidate For The Acetylation of Hepatic Carbonic Anhydrase I and III in Mice Nurdan GONUL BALTACI Ataturk University: Ataturk Universitesi Enver Fehim KOCPINAR Muş Alparslan Üniversitesi: Mus Alparslan Universitesi Harun Budak ( [email protected] ) Ataturk University https://orcid.org/0000-0002-7371-8959 Research Article Keywords: Carbonic anhydrases, Tip60, acetylation, circadian rhythm, mice Posted Date: March 22nd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-323673/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/15 Abstract Carbonic anhydrases (CAs) play an important role in maintaining pH balance by catalyzing the conversion of carbon dioxide to bicarbonate. Since this pH balance is critical to health, all organisms must develop mechanisms to control and regulate it. Although there is a great deal of literature on the biochemical, functional, and structural properties of the CA family, there is no enough knowledge on the regulation of CAs at gene and protein levels, especially their epigenetic regulation. In this study, impact of Tip60, a member of histone acetyltransferases family, on the expression of Ca1 and Ca3 genes in liver tissue was investigated at different zeitgeber time points in control and liver-specic Tip60 knockout mice (mutant) groups. First of all, Tip60 was specically knocked out in mouse liver the using Cre/loxP system and knockout rate was shown as 83% - 88% by southern blot. Expression proles of Ca1 and Ca3 genes in both groups were determined by Real-Time PCR at six different time points. While Ca1 showed the highest expression at ZT8 and ZT12, the lowest expression prole was observed at ZT0 and ZT20. -

Extracellular-Signal Regulated Kinase: a Central Molecule Driving Epithelial-Mesenchymal Transition in Cancer
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2019-06-13 Extracellular-Signal Regulated Kinase: A Central Molecule Driving Epithelial-Mesenchymal Transition in Cancer Monserrat Olea-Flores Autonomous University of Guerrero Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Cancer Biology Commons, Cell Biology Commons, Enzymes and Coenzymes Commons, and the Molecular Biology Commons Repository Citation Olea-Flores M, Zuniga-Eulogio MD, Mendoza-Catalan MA, Rodriguez-Ruiz HA, Castaneda-Saucedo E, Ortuno-Pineda C, Padilla-Benavides T, Navarro-Tito N. (2019). Extracellular-Signal Regulated Kinase: A Central Molecule Driving Epithelial-Mesenchymal Transition in Cancer. Open Access Articles. https://doi.org/10.3390/ijms20122885. Retrieved from https://escholarship.umassmed.edu/oapubs/ 3883 Creative Commons License This work is licensed under a Creative Commons Attribution 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. International Journal of Molecular Sciences Review Extracellular-Signal Regulated Kinase: A Central Molecule Driving Epithelial–Mesenchymal Transition in Cancer Monserrat Olea-Flores 1 , Miriam Daniela Zuñiga-Eulogio 1 , Miguel Angel Mendoza-Catalán 2 , Hugo Alberto Rodríguez-Ruiz 2, Eduardo Castañeda-Saucedo 1, Carlos Ortuño-Pineda 2, Teresita Padilla-Benavides 3,* and Napoleón Navarro-Tito 1,* 1 Laboratorio de Biología Celular del Cáncer, Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Guerrero, Av. -
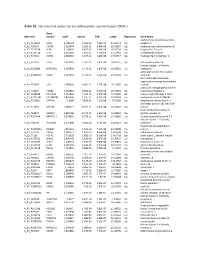
Table S1. the Statistical Metrics for Key Differentially Expressed Genes (Degs)
Table S1. The statistical metrics for key differentially expressed genes (DEGs) Gene Agilent Id Symbol logFC pValue FDR tvalue Regulation Gene Name oxidized low density lipoprotein A_24_P124624 OLR1 2.458429 1.19E-13 7.25E-10 24.04241 Up receptor 1 A_23_P90273 CHST8 2.622464 3.85E-12 6.96E-09 19.05867 Up carbohydrate sulfotransferase 8 A_23_P217528 KLF8 2.109007 4.85E-12 7.64E-09 18.76234 Up Kruppel like factor 8 A_23_P114740 CFH 2.651636 1.85E-11 1.79E-08 17.13652 Up complement factor H A_23_P34031 XAGE2 2.000935 2.04E-11 1.81E-08 17.02457 Up X antigen family member 2 A_23_P27332 TCF4 1.613097 2.32E-11 1.87E-08 16.87275 Up transcription factor 4 histone cluster 1 H1 family A_23_P250385 HIST1H1B 2.298658 2.47E-11 1.87E-08 16.80362 Up member b abnormal spindle microtubule A_33_P3288159 ASPM 2.162032 2.79E-11 2.01E-08 16.66292 Up assembly H19, imprinted maternally expressed transcript (non-protein A_24_P52697 H19 1.499364 4.09E-11 2.76E-08 16.23387 Up coding) potassium voltage-gated channel A_24_P31627 KCNB1 2.289689 6.65E-11 3.97E-08 15.70253 Up subfamily B member 1 A_23_P214168 COL12A1 2.155835 7.59E-11 4.15E-08 15.56005 Up collagen type XII alpha 1 chain A_33_P3271341 LOC388282 2.859496 7.61E-11 4.15E-08 15.55704 Up uncharacterized LOC388282 A_32_P150891 DIAPH3 2.2068 7.83E-11 4.22E-08 15.5268 Up diaphanous related formin 3 zinc finger protein 185 with LIM A_23_P11025 ZNF185 1.385721 8.74E-11 4.59E-08 15.41041 Up domain heat shock protein family B A_23_P96872 HSPB11 1.887166 8.94E-11 4.64E-08 15.38599 Up (small) member 11 A_23_P107454