Application for a Research Grant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Great British Brain Drain an Analysis of Migration to and from Manchester
The Great British Brain Drain An analysis of migration to and from Manchester Rebecca McDonald March 2019 About Centre for Cities Centre for Cities is a research and policy institute, dedicated to improving the economic success of UK cities. We are a charity that works with cities, business and Whitehall to develop and implement policy that supports the performance of urban economies. We do this through impartial research and knowledge exchange. For more information, please visit: www.centreforcities.org/about Partnerships Centre for Cities is always keen to work in partnership with like-minded organisations who share our commitment to helping cities to thrive, and supporting policymakers to achieve that aim. As a registered charity (no. 1119841) we rely on external support to deliver our programme of quality research and events. To find out more please visit: www.centreforcities.org/about/partnerships About the authors Rebecca McDonald is an Analyst at Centre for Cities: [email protected] | 0207 803 4325 Acknowledgements The authors would like to thank the University of Manchester for the support which has made this research possible. Centre for Cities • Manchester Brain Drain • March 2019 00. Executive summary Migration between Manchester and the rest of the North West region is very common. A third of those moving into the city came from the North West, and a third of those leaving Manchester stayed in the region. Overall, between 2009 and 2017 more people left the city to live elsewhere in the UK than moved in, leading to a net outflow of 31,620 people. Young people migrate to the city for university and work, while older graduates move away. -

DTA University Services Directory DTA University Services Directory
DTA University Services Directory DTA University Services Directory Contents Overview of services available at DTA Partner Institutions 3 Mental Health resources accessible to all students 3 University of Brighton 4 University of Central Lancashire 5 Coventry University 6 University of Greenwich 7 University of Hertfordshire 8 Huddersfield University 9 Kingston University 10 University of Lincoln 11 Liverpool John Moores University 12 Manchester Metropolitan University 13 Nottingham Trent University 14 Open University 15 Plymouth University 16 University of Portsmouth 17 University of Salford 18 Sheffield Hallam University 19 University of South Wales 20 Teesside University 21 Ulster University 22 University of West of England 23 Careers Unions Support 2 Doctoral Training Alliance University House, 109-117 Middlesex Street, London E1 7JF Issued November 2020 0207 839 2757 www.unialliance.ac.uk/dta/ DTA University Services Directory Overview of services available at DTA Partner Institutions All DTA host universities provide a range of services to support and enhance your doctoral training experience. These include the following: Accommodation – these links detail accommodation options provided by universities. They may also be able to offer advice about off-campus renting. Careers – all DTA institutions have Careers Centres that offer one-to-one careers guidance, support with CVs, mock interviews and careers fairs with business and industry representatives. Many also offer support for students starting their own businesses. Mental Health and Wellbeing - DTA universities offer a range of services and resources to support your wellbeing and respond to mental health difficulties. These include online resources, drop-in and bookable counselling sessions, self-guided therapy, mental health apps, self-assessments, workshops and helplines. -

University of Salford Information Sheet: Erasmus+ Exchange Partners
UNIVERSITY OF SALFORD INFORMATION SHEET: ERASMUS+ EXCHANGE PARTNERS Institution Details Name University of Salford Erasmus+ ID code UK SALFORD01 PIC Number 999829441 Professor Helen Marshall, Vice Chancellor, University of Head of Institution Salford The Crescent, Salford, Greater Manchester, M5 4WT, Address United Kingdom Website http://www.salford.ac.uk/ Staff Details Department Study Abroad and Exchanges Team Maxwell Building, Sixth Floor, The Crescent Department Address Greater Manchester, M5 4WT, United Kingdom Paul Ward, International & Regional Development Director Directorate Inez Janna International Mobility Officer Contact Person Summers [email protected] Study Abroad and Exchanges Assistant Contact Person 2 Alessandra Poti [email protected] Erasmus+ Enquiries 0161 295 2705 [email protected] Exchanges Website http://www.salford.ac.uk/study-abroad-and-exchange General Information http://www.salford.ac.uk/study/a-z- Courses courses?result_279643_result_page=A Language of Instruction English Academic Calendar https://www.salford.ac.uk/qeo/almanac Induction / Welcome One week before classes begin Exchange Information Nomination Deadlines Winter Semester - 1st April Spring Semester - 15th October Students must be nominated by their home institution using our online form - https://myadvantage.salford.ac.uk/Form.aspx?id=376938. We will pass this information on to the students’ chosen Nomination Process School for them to make a decision based on the information provided. If the nomination is accepted, this remains conditional -

Dementia Care at University of Salford 2 Salford Institute for Dementia
DEMENTIA CARE AT UNIVERSITY OF SALFORD 2 SALFORD INSTITUTE FOR DEMENTIA The Salford Institute for Dementia, based at the University of Salford, aims to improve the lived experience of people affected by dementia. Our research is focused on the challenges faced by people living with dementia and their care partners and seeks to improve the lives of these individuals in a variety of innovative ways. https://www.salford.ac.uk/salford-institute-for-dementia 3 Dementia Associates Sid’s Café At the Institute we have a panel of Dementia Memory Cafés have been found to promote Associates who act as our experts by social inclusion, prevent isolation, and experience. This panel is made up of people improve the social and emotional well-being living with dementia and their care partners. of attendees5. They are also a key source of They meet with academics from the Institute support and respite for family carer6. Cafés once a month to talk about projects and to offer a ‘safe space’ for people living with steer our research. They are also involved in dementia7 where they can meet and interact education provision and community with others who are experiencing similar engagement at the Institute. situations. It is a space where bonds of friendships are formed and valuable peer People living with dementia have reported support is offered. The Institute runs a an appetite for influencing dementia monthly memory café to provide mental specific attitudes, policy and services. stimulation, social interaction and to improve Despite this, initiatives aimed at enabling wellbeing in a communal environment. such influence are currently under- 1 researched . -

Assessment and Marking: Case Studies from Other Institutions
Title: Assessment and Marking: Case Studies from Other Institutions Date: January 2014 Author: Kevin Hewitt, Project Officer, DSE Enquiries: Kevin Hewitt, Project Officer, DSE Status: Final Introduction This report outlines the approach of a selection of other Universities to issues surrounding assessment and marking. In doing so, it gives an insight into the varieties of practice which exist across the sector and aims to inform possible future approaches at the University of Manchester. Four Universities have been chosen for the purpose of comparison – two from the Russell Group (Bristol and Liverpool) and two local Universities who have recently revised their approaches to assessment (MMU and Salford). The report concentrates on the following key issues, in line with the Project Charter for the Assessment and Marking Project: Timing, amount, structure and variety of assessment Marking and marking schemes Reassessment policies, including policies on reassessment overseas Note that this report focuses on assessment and marking for students on taught programmes. The assessment of students on research programmes is not covered here. Case Studies Case Study One – The University of Bristol The University of Bristol’s key policies on assessment can be found in their ‘Regulations and Code of Practice for Taught Programmes: Rules for Assessment, Progression and the Award of a Qualification.’1 Of all the regulations considered in this report, the University of Bristol’s are perhaps the most similar to the University of Manchester’s, but there -

The Missing Dimension- the Relevance of People's Conception Of
Edinburgh Research Explorer The missing dimension: The relevance of people's conception of time Citation for published version: Norgate, S, Davies, N, Speed, C, Cherrett, T & Dickinson, J 2014, 'The missing dimension: The relevance of people's conception of time', Behavioral and Brain Sciences, vol. 37, no. 01, pp. 93-94. https://doi.org/10.1017/S0140525X13001829 Digital Object Identifier (DOI): 10.1017/S0140525X13001829 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Behavioral and Brain Sciences Publisher Rights Statement: ©Norgate, S., Davies, N., Speed, C., Cherrett, T., & Dickinson, J. (2014). The missing dimension: The relevance of people's conception of time. Behavioral and Brain Sciences, 37(01), 93-94. 10.1017/S0140525X13001829 General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 INVITED COMMENTARY ON: R.Alexander Bentley, Michael J. O’Brien & William A. Brock >>>>CORRESPONDING AUTHOR: Sarah Norgate 1.Sarah H. Norgate, a Nigel Davies, b Chris Speed, c Tom Cherrett d and Janet Dickinson e 2. -

University of Salford and Ex Libris
Alma Impact and Value Report University of Salford and Ex Libris The University of Salford is a public university in the UK with undergraduate and post-graduate programs in the liberal arts, business and law, health, media, and the built environment. Salford serves 17,000 students on its campuses near Manchester. Salford’s three libraries have a combined staff of 81 FTE, and the collections include 350,000 books, 23,000 e-journals, and 575,000 purchased e-books. Previous ILS/Systems: Capita Alto (previously known as Talis Alto), SFX Summary of Institutional Moving to Alma Benefits: Salford’s consideration of a next department, which had its own generation library system began with priorities for upgrades and • Alma and Primo provide one-stop a proposal to the University to programming. Basic needs such as discovery and access to resources for support a digital library, including fiscal year rollover had to be planned users self-service, RFID, and a discovery months in advance, and system. The University had also enhancements for the Library • Alma gives library staff control to move invested in a campus presence at frequently waited in a queue. Salford collections and make policy changes MediaCityUK, which houses the was paying additional money for BBC, ITV, and other media-related development and consulting to • Libraries can measure their businesses. The Library needed integrate their LMS with external performance and effectiveness using systems, technologies, and systems, such as their self-check Alma Analytics collections to support the teaching at machines. They wanted to streamline this campus. to fewer systems, improve their control, and plan for the future based To leverage the potential of Primo, on the needs of their user which had already been community. -
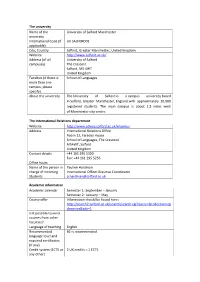
The University Name of the University University of Salford
The university Name of the University of Salford Manchester university International Code (if UK SALFORD01 applicable) City, Country Salford, Greater Manchester, United Kingdom Website http://www.salford.ac.uk/ Address (of all University of Salford campuses) The Crescent Salford, M5 4WT United Kingdom Faculties (if there is School of Languages more than one campus, please specify) About the university The University of Salford is a campus university based in Salford, Greater Manchester, England with approximately 20,000 registered students. The main campus is about 1.5 miles west of Manchester city centre. The International Relations department Website http://www.advice.salford.ac.uk/erasmus Address International Relations Office Room 12, Faraday House School of Languages, The Crescend M54WT, Salford United Kingdom Contact details +44 161 295 5320 Fax: +44 161 295 5256 Office hours Name of the person in Pauline Hardman charge of Incoming International Officer-Erasmus Coordinator Students [email protected] Academic information Academic calendar Semester 1: September – January Semester 2: January – May Course offer Information should be found here: http://search2.salford.ac.uk/search/search.cgi?query=&collection=cp dcourses&adv=1 Is it possible to enrol courses from other faculties? Language of teaching English Recommended B2 is recommended. language level and required certificates (if any) Credit system (ECTS or 2 UK credits = 1 ECTS any other) Usual work load 30 ECTS per semester. (yearly or per 60 ECTS per year. semester) Grading system A (Pass with Distinction): 70% and above B+ (Pass with Merit): 65-69% B (Pass with Merit): 60-64% C+ (Pass): 55-59% C (Pass): 50-54% D (Pass): 40-49% F (Fail): 0-39% Life abroad Housing offer Salford University has special housing for Erasmus and exchange students at Castle Irwell, at a weekly price of £76.79. -

LIVERPOOL HOPE UNIVERSITY Undergraduate Prospectus 2016
LIVERPOOL HOPE UNIVERSITY Undergraduate Prospectus 2016 YOUR FUTURE STARTS WITH HOPE Open Days Our Open Days provide potential students and their families with a great opportunity to fi nd out about life at Liverpool Hope University. On the day you will be able to get advice on: Meet the team • Your chosen course You can meet the Student • Entry criteria and applying Recruitment team at UCAS fairs and • Funding your studies other events held around the UK. • Accommodation options The team can also arrange individual • Learning support or group visits to the University or • Planning your future career. deliver workshops at schools. There will also be guided tours of For more information, please contact Hope Park and the Creative Campus the Student Recruitment team: including the sports facilities, libraries, accommodation and t: 0151 291 3111 subject-specifi c areas. e: [email protected] Most importantly, there will be the chance to meet with current students who will be able to tell you what it is like to study at Liverpool Hope University. More information is available at www.hope.ac.uk/opendays Open Day dates • Wednesday 24th June 2015 • Saturday 27th June 2015 • Saturday 12th September 2015 • Saturday 3rd October 2015 • Saturday 24th October 2015 1 Hope Park 1 Gateway Building 2 EDEN Building 3 Hilda Constance Allen Building 4 Sheppard–Worlock Library 5 Residential Accommodation 6 Lecture Theatre Complex 7 Sports Hall 8 Business School 9 New Science Building for 2016 9 7 5 4 STAND PARK ROAD 3 TAGGART AVENUE 15 2 HOPE PARK TAGGART AVENUE -

LATEST SHORTHAND RESULTS Please Note: 1. Results Can Take Up
LATEST SHORTHAND RESULTS Please note: 1. Results can take up to four weeks before they appear in the student log-in area. 2. Certificates will be sent to exam centres within 5 weeks of the exam. 3. We are unable to give results over the phone. EXAM DATE: 28 MAY Centres Speeds Cardiff University 100 Darlington College 100 Falmouth University 100 Gloucestershire Live 100 Highbury College 100 Huntingdon Post 100 Kingston University 100 120 Leeds Trinity University 100 News Associates London 100 News Associates Manchester 100 Nottingham Trent University 100 PA Training 100 120 Sutton College 100 University of Lincoln 100 University of Salford 100 100wpm Bannister, Samuel Pass Bellis, Richard Pass Brougham, Mhari Pass. 100% accuracy Carr, Megan Pass Clark, Thomas Pass Cook, Julia Pass Cook, Rebecca Pass Curston, Naomi Pass Duffy, Muireann Pass Gallop, Joseph Alexander Pass Gray, Molly May Pass Halliday, Ellen Pass Hanaphy, Paul Pass Holmes, Tom Pass Hyde, Davina Pass Johnson, Calum Pass. 100% accuracy Jones, Harry Pass Maccana, Laoishe Pass Madeira, Luke Pass Newton, Francesca Pass Noye-Allen, Rhys Pass Osterloh, Zoe Pass Parkinson, Anna Pass Parry, Isaac Pass Percy, Hamish Pass. 100% accuracy Post, Christopher Pass Pounds, Kate Pass Poysner, Joshua Pass Pratt McCullagh, Dylan Pass Price, Oliver Pass Ross, Alex Pass Sajan, Kiran Tom Pass Salley, Emily Pass Shahaney, Pranev Pass Sharman, Katie Pass Talora, Giuseppe Benjamin Pass Telling, Oliver Pass Thompson, Becky Pass 120wpm Moloney, Charlie Pass. 100% accuracy end. EXAM DATE: 29 MAY Centres -

University of Salford
Accomplishing social work identity in interprofessional mental health teams following the implementation of the Mental Health Act 2007 Lisa Morriss Thesis submitted in candidacy for the degree of Doctor of Philosophy University of Salford Department of Social Work School of Nursing, Midwifery and Social Work 2014 Table of Contents List of tables ............................................................................................................. viii Acknowledgements .................................................................................................... ix List of abbreviations .................................................................................................... x Abstract ..................................................................................................................... xi Introduction ................................................................................................................ 1 Objectives of the research ........................................................................................... 4 1 A Natural History Approach to the Methodology Chapter ................................... 5 1.1 A Natural History Approach .............................................................................. 5 1.2 Being a mental health social worker ................................................................. 5 1.3 Interviews: “semi-structured?” ......................................................................... 6 1.4 The literature review........................................................................................ -

Spark: UAL Creative Teaching and Learning Journal
Vol 1 / Issue 1 (2016) pp. 46–48 Spark: UAL Creative Teaching and Learning Journal Event review of ‘De-Brief: Interrogating the Graphic Design Brief’ at University of Salford, 9th September 2015 Emily Wood, Lecturer, BA (Hons) Graphic Design, Central St Martins Abstract Event review of ‘De-Brief: Interrogating the Graphic Design Brief’, the inaugural symposium of the Graphic Design Educators’ Network, a new subject association, held at University of Salford on 9th September 2015. Keywords graphic design, subject associations, networks, the brief, Graphic Design Educators’ Network Before the frenzied start of the new academic year, 80 or so graphic design lecturers met at the University of Salford for the inaugural meeting of the Graphic Design Educators’ Network (GDEN). This initiative was started by a group of ten graphic design lecturers from different universities across the UK, with the aim of inciting discourse about our practices, and was initiated by this one-day symposium entitled ‘De-brief: Interrogating the Graphic Design Brief’. Figure 1: Photo by Paul Bailey, Graphic Design Educators’ Network (GDEN), Wednesday 9 September 2015, University of Salford. © 2016 Spark: UAL Creative Teaching and Learning Journal 46 Spark: UAL Creative Teaching and Learning Journal / Vol 1 / Issue 1 (2016) Event review of ‘De-Brief: Interrogating the Graphic Design Brief’ This symposium presented the opportunity to explore the breadth of graphic design courses currently being run in the UK and consider how the subject is taught around the country. It interrogated ‘The Brief’ as both the start of a graphic design project and the beginning of the GDEN’s formation.