Seagrass Ecosystems of India As Bioindicators of Trace Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
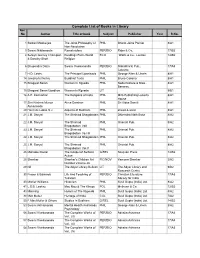
Complete List of Books in Library Acc No Author Title of Book Subject Publisher Year R.No
Complete List of Books in Library Acc No Author Title of book Subject Publisher Year R.No. 1 Satkari Mookerjee The Jaina Philosophy of PHIL Bharat Jaina Parisat 8/A1 Non-Absolutism 3 Swami Nikilananda Ramakrishna PER/BIO Rider & Co. 17/B2 4 Selwyn Gurney Champion Readings From World ECO `Watts & Co., London 14/B2 & Dorothy Short Religion 6 Bhupendra Datta Swami Vivekananda PER/BIO Nababharat Pub., 17/A3 Calcutta 7 H.D. Lewis The Principal Upanisads PHIL George Allen & Unwin 8/A1 14 Jawaherlal Nehru Buddhist Texts PHIL Bruno Cassirer 8/A1 15 Bhagwat Saran Women In Rgveda PHIL Nada Kishore & Bros., 8/A1 Benares. 15 Bhagwat Saran Upadhya Women in Rgveda LIT 9/B1 16 A.P. Karmarkar The Religions of India PHIL Mira Publishing Lonavla 8/A1 House 17 Shri Krishna Menon Atma-Darshan PHIL Sri Vidya Samiti 8/A1 Atmananda 20 Henri de Lubac S.J. Aspects of Budhism PHIL sheed & ward 8/A1 21 J.M. Sanyal The Shrimad Bhagabatam PHIL Dhirendra Nath Bose 8/A2 22 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam VolI 23 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam Vo.l III 24 J.M. Sanyal The Shrimad Bhagabatam PHIL Oriental Pub. 8/A2 25 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam Vol.V 26 Mahadev Desai The Gospel of Selfless G/REL Navijvan Press 14/B2 Action 28 Shankar Shankar's Children Art FIC/NOV Yamuna Shankar 2/A2 Number Volume 28 29 Nil The Adyar Library Bulletin LIT The Adyar Library and 9/B2 Research Centre 30 Fraser & Edwards Life And Teaching of PER/BIO Christian Literature 17/A3 Tukaram Society for India 40 Monier Williams Hinduism PHIL Susil Gupta (India) Ltd. -

The Story of India by Young Writers
Annual Subscription Rs 5.00; 50 paise per copy March 2021 • Vol 39 No. 3 Contents The Story of India by Young Writers The Story of India by ndia is the third largest publisher of about a complete change in a person’s Young Writers 1-2 Ibooks in the world. This despite the fact life? There are many examples in the that only a small number of people adopt history of our country where such changes Netaji National Reading Promotion writing as a profession in our country. were brought about by words. India will Recognition Award The strength of a country’s defence, nurture children from their early years 2 health, transport and communication, and the youth in a way that they will Azamgarh Book Fair which are seen as symbols of a country’s be able to become ambassadors of its 2 empowerment becomes meaningful, only literature of a twenty-first century India. if its people are able to express their For every Indian to be a “world citizen”, it In Conversation 3 achievements, aspirations and hopes in is imperative that the voice of the country their own words and languages. How does is heard in their own language on a global Lifetime Achievement Award the reading of a book or an event bring stage in a structured manner. to NBT 3 NBT Books on and by Women 4-5 Excerpts 6 Celebrate World Wildlife Day with NBT Books 7 PICK OF THE MONTH Pleasures of Reading Prem Pal Sharma Translation: Arpita Sen ISBN 978-237-81-9523-2; Rs 175 MARCH 2021 NBT NEWSLETTER 1 As you may be aware, on 31 January Prime Minister will create a large number society rooted in the objectives of the 2021 in his weekly broadcast programme of young writers, who will able to express National Education Policy-2020, will of “Mann Ki Baat”, the Prime Minister themselves in their own mother tongue promote Indian languages and their said, “In every corner of the land, and their writings will be promoted with literatures among the youth. -

The Current Status of Halophila Beccarii: an Ecologically Significant, Yet Vulnerable
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 5 August 2020 doi:10.20944/preprints202008.0126.v1 The current status of Halophila beccarii: An ecologically significant, yet Vulnerable seagrass of India 1Amrit Kumar Mishra, 1Deepak Apte 1Marine Conservation Department, Bombay Natural History Society, Hornbill House, Dr. Salim Ali Chowk, Shaheed Bhagat Singh Road, Opp. Lion Gate, Mumbai, 400001, India Corresponding author: [email protected] Abstract: We reviewed the current status of a Vulnerable seagrass, Halophila beccarii from the coast of India using the published data from 1977-2020. We found that the seagrass, H. beccarii has a pan India distribution on both east and west coast. It is abundant in the intertidal silty-muddy region on the west coast, while on the east coast it is found on sandy habitats, with few exceptions of muddy habitat. H. beccarii was found to be associated with mangroves or smaller seagrass species within a depth limit of 1.7m. Low salinity and high nitrate levels were observed for the H. beccarii meadows of the west coast due to its association with mangroves. The nutrient levels in H. beccarii meadows of India were comparatively lower than other seagrass meadows. Most of the research on H. beccarii has focoused on its morphometrics (41%), reproductive (33%) and distribution (29%) along the coast of India. Reproductive traits such as flowering and fruiting varying according to the seasons of each coast due to the influence of monsoon and its associated temperature, salinity and nutrient influx. H. beccarii has a great potential of various bioactive compounds, which needs further investigation. -

Library Catalogue
Id Access No Title Author Category Publisher Year 1 9277 Jawaharlal Nehru. An autobiography J. Nehru Autobiography, Nehru Indraprastha Press 1988 historical, Indian history, reference, Indian 2 587 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence historical, Indian history, reference, Indian 3 605 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence 4 3633 Jawaharlal Nehru. Rebel and Stateman B. R. Nanda Biography, Nehru, Historical Oxford University Press 1995 5 4420 Jawaharlal Nehru. A Communicator and Democratic Leader A. K. Damodaran Biography, Nehru, Historical Radiant Publlishers 1997 Indira Gandhi, 6 711 The Spirit of India. Vol 2 Biography, Nehru, Historical, Gandhi Asia Publishing House 1975 Abhinandan Granth Ministry of Information and 8 454 Builders of Modern India. Gopal Krishna Gokhale T.R. Deogirikar Biography 1964 Broadcasting Ministry of Information and 9 455 Builders of Modern India. Rajendra Prasad Kali Kinkar Data Biography, Prasad 1970 Broadcasting Ministry of Information and 10 456 Builders of Modern India. P.S.Sivaswami Aiyer K. Chandrasekharan Biography, Sivaswami, Aiyer 1969 Broadcasting Ministry of Information and 11 950 Speeches of Presidente V.V. Giri. Vol 2 V.V. Giri poitical, Biography, V.V. Giri, speeches 1977 Broadcasting Ministry of Information and 12 951 Speeches of President Rajendra Prasad Vol. 1 Rajendra Prasad Political, Biography, Rajendra Prasad 1973 Broadcasting Eminent Parliamentarians Monograph Series. 01 - Dr. Ram Manohar 13 2671 Biography, Manohar Lohia Lok Sabha 1990 Lohia Eminent Parliamentarians Monograph Series. 02 - Dr. Lanka 14 2672 Biography, Lanka Sunbdaram Lok Sabha 1990 Sunbdaram Eminent Parliamentarians Monograph Series. 04 - Pandit Nilakantha 15 2674 Biography, Nilakantha Lok Sabha 1990 Das Eminent Parliamentarians Monograph Series. -

Stamps of India - Commemorative by Prem Pues Kumar [email protected] 9029057890
E-Book - 26. Checklist - Stamps of India - Commemorative By Prem Pues Kumar [email protected] 9029057890 For HOBBY PROMOTION E-BOOKS SERIES - 26. FREE DISTRIBUTION ONLY DO NOT ALTER ANY DATA ISBN - 1st Edition Year - 1st May 2020 [email protected] Prem Pues Kumar 9029057890 Page 1 of 76 Nos. YEAR PRICE NAME Mint FDC B. 1 2 3 1947 1 21-Nov-47 31/2a National Flag 2 15-Dec-47 11/2a Ashoka Lion Capital 3 15-Dec-47 12a Aircraft 1948 4 29-May-48 12a Air India International 5 15-Aug-48 11/2a Mahatma Gandhi 6 15-Aug-48 31/2a Mahatma Gandhi 7 15-Aug-48 12a Mahatma Gandhi 8 15-Aug-48 10r Mahatma Gandhi 1949 9 10-Oct-49 9 Pies 75th Anni. of Universal Postal Union 10 10-Oct-49 2a -do- 11 10-Oct-49 31/2a -do- 12 10-Oct-49 12a -do- 1950 13 26-Jan-50 2a Inauguration of Republic of India- Rejoicing crowds 14 26-Jan-50 31/2a Quill, Ink-well & Verse 15 26-Jan-50 4a Corn and plough 16 26-Jan-50 12a Charkha and cloth 1951 17 13-Jan-51 2a Geological Survey of India 18 04-Mar-51 2a First Asian Games 19 04-Mar-51 12a -do- 1952 20 01-Oct-52 9 Pies Saints and poets - Kabir 21 01-Oct-52 1a Saints and poets - Tulsidas 22 01-Oct-52 2a Saints and poets - MiraBai 23 01-Oct-52 4a Saints and poets - Surdas 24 01-Oct-52 41/2a Saints and poets - Mirza Galib 25 01-Oct-52 12a Saints and poets - Rabindranath Tagore 1953 26 16-Apr-53 2a Railway Centenary 27 02-Oct-53 2a Conquest of Everest 28 02-Oct-53 14a -do- 29 01-Nov-53 2a Telegraph Centenary 30 01-Nov-53 12a -do- 1954 31 01-Oct-54 1a Stamp Centenary - Runner, Camel and Bullock Cart 32 01-Oct-54 2a Stamp Centenary -

Directory of Development Organizations
EDITION 2007 VOLUME II.A / ASIA AND THE MIDDLE EAST DIRECTORY OF DEVELOPMENT ORGANIZATIONS GUIDE TO INTERNATIONAL ORGANIZATIONS, GOVERNMENTS, PRIVATE SECTOR DEVELOPMENT AGENCIES, CIVIL SOCIETY, UNIVERSITIES, GRANTMAKERS, BANKS, MICROFINANCE INSTITUTIONS AND DEVELOPMENT CONSULTING FIRMS Resource Guide to Development Organizations and the Internet Introduction Welcome to the directory of development organizations 2007, Volume II: Asia and the Middle East The directory of development organizations, listing 51.500 development organizations, has been prepared to facilitate international cooperation and knowledge sharing in development work, both among civil society organizations, research institutions, governments and the private sector. The directory aims to promote interaction and active partnerships among key development organisations in civil society, including NGOs, trade unions, faith-based organizations, indigenous peoples movements, foundations and research centres. In creating opportunities for dialogue with governments and private sector, civil society organizations are helping to amplify the voices of the poorest people in the decisions that affect their lives, improve development effectiveness and sustainability and hold governments and policymakers publicly accountable. In particular, the directory is intended to provide a comprehensive source of reference for development practitioners, researchers, donor employees, and policymakers who are committed to good governance, sustainable development and poverty reduction, through: the -

Biography of Salim Ali
Biography of Salim Ali Salim Ali Salim Moizuddin Abdul Ali (12 November 1896 – 20 June 1987) was an Indian ornithologist and naturalist. Sometimes referred to as the "birdman of India", Salim Ali was among the first Indians to conduct systematic bird surveys across India and his bird books helped develop ornithology. He became the key figure behind the Bombay Natural History Society after 1947 and used his personal influence to garner government support for the organisation, create the Bharatpur bird sanctuary (Keoladeo National Park) and prevent the destruction of what is now the Silent Valley National Park. He was awarded the Padma Bhushan in 1958 and the Padma Vibhushan in 1976, India's third and second highest civilian honours respectively. Salim Ali was born into a Sulaimani Bohra Muslim family of Bombay, the ninth and youngest child. His father Moizuddin died when he was one year old and his mother Zeenat-un-nissa died when he was three. The children were brought up by his maternal uncle, Amiruddin Tyabji, and childless aunt, Hamida Begum, in a middle-class household in Khetwadi, Mumbai. Another uncle was Abbas Tyabji, well known Indian freedom fighter. Salim was introduced to the serious study of birds by W. S. Millard, secretary of the Bombay Natural History Society (BNHS), who identified an unusually coloured sparrow that young Salim had shot for sport with his toy air gun. Millard identified it as a yellow-throated sparrow, and showed Salim around the Society's collection of stuffed birds. Millard lent Salim a few books including Eha's Common birds of Bombay, encouraged Salim to make a collection of birds and offered to train him in skinning and preservation. -

Term Loan Beneficiary Wise Utilisation Report from 20.01.16 to 03.03.16
Term Loan UC report from 20.01.16 to 03.03.16 Sanction Communit Amount of NMDFC Slno Name beneficiary_cd Father's/Husband's Name Address Pin Code District Sector Gender Area Letter Print y Finance(Rs) Share(Rs) Date ABUJAR HOSSAIN A56028/MBD/58/16 SOFIKUL ISLAM VILL- NAMO CHACHANDA, PO- MURSHIDABA 1 742224 CLOTH SHOP MUSLIM MALE RURAL 80,000 72,000 12/02/2016 JOYKRISHNAPUR, PS- SAMSERGANJ D ARSAD ALI A56037/MBD/55/16 LATE MD MOSTAFA VILL- JOYRAMPUR, PO- BHABANIPUR, PS- MURSHIDABA WOODEN 2 742202 MUSLIM MALE RURAL 50,000 45,000 12/02/2016 FARAKKA D FURNITURE SHOP ABU TALEB AHAMED A56040/CBR/57/16 MAHIRUDDIN AHAMED VILL CHHATGENDUGURI PO STATIONARY 3 736159 COOCH BEHAR MUSLIM MALE RURAL 70,000 63,000 02/03/2016 KASHIRDANGA SHOP ABDUL MANNAN A56042/CBR/58/16 SABER ALI VILL- NABANI, PO- GITALDAHA , PS- SEASONAL 4 736175 COOCH BEHAR MUSLIM MALE RURAL 80,000 72,000 24/02/2016 DINAHTA CROPS TRADING MISTANNA ATAUR RAHAMAN A56101/DDP/58/16 LATE ACHIMADDIN AHAMEDVILL BARAIDANGA PO KALIMAMORA DAKSHIN 5 733132 VANDAR (SWEET MUSLIM MALE RURAL 80,000 72,000 13/02/2016 DINAJPUR SHOP) AMINUR RAHAMAN A56110/DDP/59/16 AAIDUR RAHAMAN JHANJARI PARA DAKSHIN 6 733125 GARMENTS SHOP MUSLIM MALE RURAL 90,000 81,000 13/02/2016 DINAJPUR ANARUL HAQUE A56131/NDA/60/16 NURUL ISLAM SK VILL. CHAPRA BUS STAND PO. BANGALJHI 7 741123 NADIA MEDICINE SHOP MUSLIM MALE RURAL 100,000 90,000 15/02/2016 AKIMUDDIN A56206/PRL/52A/16 KHALIL CHOWDHURY VILL+PO- KARKARA, PS- JOYPUR 8 723213 PURULIA GENERAL STORE MUSLIM MALE RURAL 25,000 22,500 13/02/2016 CHOWDHURY ARIF KAZI A56209/PRL/52A/16 LALU KAZI VILL- KANTADI, PO+PS- JHALDA 9 723202 PURULIA FURNITURE SHOP MUSLIM MALE URBAN 25,000 22,500 13/02/2016 ANUAR ALI A56282/UDP/58/16 GUFARUDDIN PRADHAN VILL- BHADRATHA, PO- BHUPAL PUR, PS- UTTAR HARDWARE 10 733143 MUSLIM MALE RURAL 80,000 72,000 24/02/2016 ITAHAR DINAJPUR SHOP ANASARUL SEKH A56432/BBM/53/16 EMDADUL ISLAM VILL BAHADURPU 11 731219 BIRBHUM GROCERY SHOP MUSLIM MALE RURAL 30,000 27,000 17/02/2016 ANISH GOLDAR A56467/HWH/55/16 LATE. -

Padma Vibhushan * * the Padma Vibhushan Is the Second-Highest Civilian Award of the Republic of India , Proceeded by Bharat Ratna and Followed by Padma Bhushan
TRY -- TRUE -- TRUST NUMBER ONE SITE FOR COMPETITIVE EXAM SELF LEARNING AT ANY TIME ANY WHERE * * Padma Vibhushan * * The Padma Vibhushan is the second-highest civilian award of the Republic of India , proceeded by Bharat Ratna and followed by Padma Bhushan . Instituted on 2 January 1954, the award is given for "exceptional and distinguished service", without distinction of race, occupation & position. Year Recipient Field State / Country Satyendra Nath Bose Literature & Education West Bengal Nandalal Bose Arts West Bengal Zakir Husain Public Affairs Andhra Pradesh 1954 Balasaheb Gangadhar Kher Public Affairs Maharashtra V. K. Krishna Menon Public Affairs Kerala Jigme Dorji Wangchuck Public Affairs Bhutan Dhondo Keshav Karve Literature & Education Maharashtra 1955 J. R. D. Tata Trade & Industry Maharashtra Fazal Ali Public Affairs Bihar 1956 Jankibai Bajaj Social Work Madhya Pradesh Chandulal Madhavlal Trivedi Public Affairs Madhya Pradesh Ghanshyam Das Birla Trade & Industry Rajashtan 1957 Sri Prakasa Public Affairs Andhra Pradesh M. C. Setalvad Public Affairs Maharashtra John Mathai Literature & Education Kerala 1959 Gaganvihari Lallubhai Mehta Social Work Maharashtra Radhabinod Pal Public Affairs West Bengal 1960 Naryana Raghvan Pillai Public Affairs Tamil Nadu H. V. R. Iyengar Civil Service Tamil Nadu 1962 Padmaja Naidu Public Affairs Andhra Pradesh Vijaya Lakshmi Pandit Civil Service Uttar Pradesh A. Lakshmanaswami Mudaliar Medicine Tamil Nadu 1963 Hari Vinayak Pataskar Public Affairs Maharashtra Suniti Kumar Chatterji Literature -
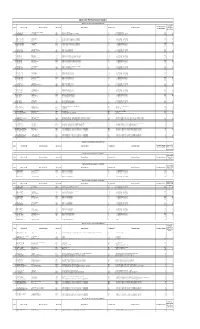
Operator Performance Report-1 (OPPR-1) Count of Checked Packets S
Operator Perfromance Report Operator Performance Report-1 (OPPR-1) Count of Checked Packets S. No. Operator ID Operator Name Agency ID Agency Name Registrar ID Registrar Name Demo Errors (in last month) 30 or More 1 MH_MOL_LA_NS013609 Shivshankar Baswaraj Balagide 2006 Mahaonline Limited 127 Govt of Maharashtra 654 34 2 MH_MOL_LAT_NS226937 Halighongde Deepak Umakant 2006 Mahaonline Limited 127 Govt of Maharashtra 787 32 3 NRCGLT423207 Layashree Bora 2777 Office of the Deputy Commissioner, Golaghat 174 Home & Political, Govt. of Assam 641 55 4 NRCJRT397933 Bhargob Doley 2778 Deputy commissioner Jorhat 174 Home & Political, Govt. of Assam 676 53 5 NLBNRC_411161 Priyangshu Sarma 2785 Deputy Commissioner Nalbari 174 Home & Political, Govt. of Assam 349 80 6 BARNPR_235007 Nabajit Das 2786 Office of the Deputy Commissioner, Barpeta 174 Home & Political, Govt. of Assam 752 67 7 BNGN_CSPL_0005 Diganta Kr Ray 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 854 45 8 BNGN_CSPL_0027 Sanjay Sarkar 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 878 74 9 BNGN_CSPL_0030 Abdul Hoque 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 572 55 10 BNGN_CSPL_0031 Saiful Islam 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 808 51 11 BNGN_CSPL_0036 Moynul Hoque 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 955 141 12 BNGN_CSPL_0037 Hamed Ali 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. of Assam 766 47 13 BNGN_CSPL_0041 Rafikul Islam 2790 Office of the Deputy Commissioner , Bongaigaon 174 Home & Political, Govt. -

July2016 Newsletter
Volume 8 issue 1 Vidya Vanam Newsletter - January 2016 2007 2009 Contents Highlights 1 2011 2015 Sports day 2 Picture Gallery 3 Project Day 4 Picture Gallery 5 Dance & Music 6 Science Block 7 Field Trips 8,9 Awards 10,11 Visitors 12 Vidya Vanam 2007—2015. A barren piece of land (2007) now has 284 different varieties of plants trees and shrubs. Highlights of 1st and 2nd Term Vanamahotsav was celebrated to respect and honor the trees and vegetaon in and around Vidya Vanam. Construcon of the new Science block began September 2015 and completed January 2016. Students completed field trips to Mysore and Sriranga‐ panam Two students were honored for their essays on ecology and conservaon Prema Rangachary was honored by the Times of India for her leadership in educaon of tribal and underprivileged children Pankaj Sekhsaria was the honored chief guest for Project Day th India Independence Day and Sports Day August 15 2015 Page 4 As always, Vidya Vanam’s sports day was held on August 15, 2015. This year, our Chief Guest was Mr. V. Baskaran, former captain of the Indian hockey team that won the gold at the 1980 Olympics. Aer the march past, the whole school assembled before the flagpole. Once the chief guest hoisted the tricolour, the children sang thaayin mani kodi and then took the oath. As it was also India’s independence day, the children presented a tableau featuring the lead‐ ers of Indian independence such as Subramania Bharathi, Dr. Ambedkar, Lakshmi Sehgal, Mahatma Gandhi and Rabindranath Tagore. Once the cultural events were over, Mr. -

CLASS 7 Ques
Section A : Current Affairs 1. Where was the second world orange festival 2019 held? A. Nasik B. Nagpur C. Pune D. Mandi 2. Who has become the youngest person in the world at the age of 35 years, 274 days to scale tallest mountains and volcanoes in all seven continents? A. Satyarup Sidhanta B. Vihwas Nigam C. Abhilash Tomy D. Abhinav Shaw 3. What is the name of the Space X‟s spacecraft that will carry people on Moon and Mars in the future? A. Newworld Falcon B. Spaceworld Falcon C. Starship Hopper D. Spaceworld Ship 4. Who gets the Dronacharya Award? A. Best wrestler B. Best batsman C. Best tennis Player D. Best coach 5. Who among the following has been selected for Padam Shri award 2019? A. Bachendri Pal B. Gautam Gambhir C. Teejan Bai D. Nambi Narayan 6. The Constitution (103rd Amendment) Act, 2019, this Act provides ____ % reservation in government jobs and educational institutions to Economically Weaker Sections (EWS) of General Category. A. 10% B. 8% C. 7% D. 5% 7. Which animated movie got the Academy Award for Best Animated Feature Film in Oscars 2018? A. The Book of Life B. Moana C. Coco D. The Grinch 8. Which football club won the UEFA Champions league 2018? A. Real Madrid B. FC Barcelona C. Liverpool FC D. Juventus IFGCO-2019/CLASS-7 PAGE NO 1 9. Who won Femina Miss India 2018? A. Pooja Chand B. Shreya Rao C. Anukreethy Vas D. Meenakshi Choudhary 10. Who among the following was a space research scientist? A.