The Current Status of Halophila Beccarii: an Ecologically Significant, Yet Vulnerable
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
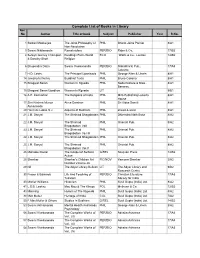
Complete List of Books in Library Acc No Author Title of Book Subject Publisher Year R.No
Complete List of Books in Library Acc No Author Title of book Subject Publisher Year R.No. 1 Satkari Mookerjee The Jaina Philosophy of PHIL Bharat Jaina Parisat 8/A1 Non-Absolutism 3 Swami Nikilananda Ramakrishna PER/BIO Rider & Co. 17/B2 4 Selwyn Gurney Champion Readings From World ECO `Watts & Co., London 14/B2 & Dorothy Short Religion 6 Bhupendra Datta Swami Vivekananda PER/BIO Nababharat Pub., 17/A3 Calcutta 7 H.D. Lewis The Principal Upanisads PHIL George Allen & Unwin 8/A1 14 Jawaherlal Nehru Buddhist Texts PHIL Bruno Cassirer 8/A1 15 Bhagwat Saran Women In Rgveda PHIL Nada Kishore & Bros., 8/A1 Benares. 15 Bhagwat Saran Upadhya Women in Rgveda LIT 9/B1 16 A.P. Karmarkar The Religions of India PHIL Mira Publishing Lonavla 8/A1 House 17 Shri Krishna Menon Atma-Darshan PHIL Sri Vidya Samiti 8/A1 Atmananda 20 Henri de Lubac S.J. Aspects of Budhism PHIL sheed & ward 8/A1 21 J.M. Sanyal The Shrimad Bhagabatam PHIL Dhirendra Nath Bose 8/A2 22 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam VolI 23 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam Vo.l III 24 J.M. Sanyal The Shrimad Bhagabatam PHIL Oriental Pub. 8/A2 25 J.M. Sanyal The Shrimad PHIL Oriental Pub. 8/A2 Bhagabatam Vol.V 26 Mahadev Desai The Gospel of Selfless G/REL Navijvan Press 14/B2 Action 28 Shankar Shankar's Children Art FIC/NOV Yamuna Shankar 2/A2 Number Volume 28 29 Nil The Adyar Library Bulletin LIT The Adyar Library and 9/B2 Research Centre 30 Fraser & Edwards Life And Teaching of PER/BIO Christian Literature 17/A3 Tukaram Society for India 40 Monier Williams Hinduism PHIL Susil Gupta (India) Ltd. -

New Record of the Seagrass Species Halophila Major (Zoll.) Miquel in Vietnam: Evidence from Leaf Morphology and ITS Analysis
DOI 10.1515/bot-2012-0188 Botanica Marina 2013; 56(4): 313–321 Nguyen Xuan Vy*, Laura Holzmeyer and Jutta Papenbrock New record of the seagrass species Halophila major (Zoll.) Miquel in Vietnam: evidence from leaf morphology and ITS analysis Abstract: The seagrass Halophila major (Zoll.) Miquel (i) the number of cross veins, which ranges from 18 to 22, is reported for the first time from Vietnam. It was found and (ii) the ratio of the distance between the intramar- growing with other seagrass species nearshore, 4–6 m ginal vein and the lamina margin at the half-way point deep at Tre Island, Nha Trang Bay. Leaf morphology and along the leaf length, which is 1:20–1:25 (Kuo et al. 2006). phylogenetic analysis based on ribosomal internal tran- Recently, genetic markers, including plastid and nuclear scribed spacer sequences confirmed the identification. sequences, have been used to reveal the genetic relation- There was very little sequence differentiation among sam- ships among members of the genus Halophila. Among the ples of H. major collected in Vietnam and other countries molecular markers used, neither single sequence analysis in the Western Pacific region. A very low evolutionary of the plastid gene encoding the large subunit of ribulose- divergence among H. major populations was found. 1,5-bisphosphate-carboxylase-oxygenase (rbcL) and of the plastid maturase K (matK) nor analysis of the concat- Keywords: Halophila major; internal transcribed spacer; enated sequences of the two plastid markers has resolved new record; seagrass; Vietnam. the two closely related species H. ovalis and H. ovata (Lucas et al. -

The 16Th International Congress on Marine Corrosion and Fouling
16th Annual International Congress on Marine Corrosion and Fouling ABSTRACT SUMMARIES The 16th International Congress on Marine Corrosion and Fouling 24-28- June 2012 in Seattle, Washington ORGANIZED ON BEHALF OF COMITÉ INTERNATIONAL PERMANENT PUR LA RECHERCHE SU LA PRÉSERVATION DES MATÉRIAUX EN MILIEU MARIN (COIPM) The COIPM Committee consists of: Chair 2010-2012: . Geoff Swain Florida Institute of Technology Secretary and Vice Chair: . Andrew Scardino Defence Science and Technology Organisation Treasurer: . Claire Hellio University of Portsmouth Committee Members: . Maureen Callow University of Birmingham . Tony Clare Newcastle University . Ricardo Coutinho Instituto de Estudos do Mar Almirante Paulo Moreira . Brenda Little Naval Research Laboratory . Steve McElvany Office of Naval Research . Marianne Pereira Hempel . Kiyoshi Shibata Chiba Institute of Technology . Serena Teo National University of Singapore . David Williams International Paint, Ltd Student Committee: . Emily Ralston Florida Institute of Technology . Sheelagh Conlan Liverpool John Moores University i ABSTRACT SUMMARIES 16th Annual International Congress on Marine Corrosion and Fouling Organization Congress Chairman: . Steve McElvany The Office of Naval Research Local Organizing Committee: . Geoff Swain Florida Institute of Technology . Linda Chrisey The Office of Naval Research . Paul Armistead The Office of Naval Research . Shaoyi Jiang The University of Washington . Brenda Little Naval Research Laboratory . Kristin Grehawick Strategic Analysis, Inc. Anthony Brennan -

Global Seagrass Distribution and Diversity: a Bioregional Model ⁎ F
Journal of Experimental Marine Biology and Ecology 350 (2007) 3–20 www.elsevier.com/locate/jembe Global seagrass distribution and diversity: A bioregional model ⁎ F. Short a, , T. Carruthers b, W. Dennison b, M. Waycott c a Department of Natural Resources, University of New Hampshire, Jackson Estuarine Laboratory, Durham, NH 03824, USA b Integration and Application Network, University of Maryland Center for Environmental Science, Cambridge, MD 21613, USA c School of Marine and Tropical Biology, James Cook University, Townsville, 4811 Queensland, Australia Received 1 February 2007; received in revised form 31 May 2007; accepted 4 June 2007 Abstract Seagrasses, marine flowering plants, are widely distributed along temperate and tropical coastlines of the world. Seagrasses have key ecological roles in coastal ecosystems and can form extensive meadows supporting high biodiversity. The global species diversity of seagrasses is low (b60 species), but species can have ranges that extend for thousands of kilometers of coastline. Seagrass bioregions are defined here, based on species assemblages, species distributional ranges, and tropical and temperate influences. Six global bioregions are presented: four temperate and two tropical. The temperate bioregions include the Temperate North Atlantic, the Temperate North Pacific, the Mediterranean, and the Temperate Southern Oceans. The Temperate North Atlantic has low seagrass diversity, the major species being Zostera marina, typically occurring in estuaries and lagoons. The Temperate North Pacific has high seagrass diversity with Zostera spp. in estuaries and lagoons as well as Phyllospadix spp. in the surf zone. The Mediterranean region has clear water with vast meadows of moderate diversity of both temperate and tropical seagrasses, dominated by deep-growing Posidonia oceanica. -

Reassessment of Seagrass Species in the Marshall Islands1
Micronesica 2016-04: 1–10 Reassessment of Seagrass Species in the Marshall Islands 1 ROY T. TSUDA Department of Natural Sciences, Bishop Museum, 1525 Bernice Street, Honolulu, HI 96817, USA [email protected] NADIERA SUKHRAJ U.S. Fish and Wildlife Service, Pacific Islands Fish and Wildlife Office, 300 Ala Moana Blvd., Honolulu, HI 96850, USA [email protected] Abstract—Recent collections of specimens of Halophila gaudichaudii J. Kuo, previously identified as Halophila minor (Zollinger) den Hartog, from Kwajalein Atoll in September 2016 and the archiving of the specimens at BISH validate the previous observation of this seagrass genus in the Marshall Islands. Previously, no voucher specimen was available for examination. Molecular analyses of the Kwajalein Halophila specimens may demonstrate conspecificity with Halophila nipponica J. Kuo with H. gaudichaudii relegated as a synonym. Herbarium specimens of Cymodocea rotundata Ehrenberg and Hemprich ex Ascherson from Majuro Atoll were found at BISH and may represent the only specimens from the Marshall Islands archived in a herbarium. Cymodocea rotundata, however, has been documented in past literature and archived via digital photos in its natural habitat in Majuro. The previous validation of Thalassia hemprichii (Ehrenberg) Ascherson with specimens, and the recent validation of Halophila gaudichaudii and Cymodocea rotundata with specimens reaffirm the low coral atolls and islands of the Marshall Islands as the eastern limit for the three species in the Pacific Ocean. Introduction In a review of the seagrasses in Micronesia, Tsuda et al. (1977) reported nine species of seagrasses in Micronesia with new records of Thalassodendron ciliatum (Forsskål) den Hartog from Palau, and Syringodium isoetifolium (Ascherson) Dandy and Cymodocea serrulata (R. -

The Story of India by Young Writers
Annual Subscription Rs 5.00; 50 paise per copy March 2021 • Vol 39 No. 3 Contents The Story of India by Young Writers The Story of India by ndia is the third largest publisher of about a complete change in a person’s Young Writers 1-2 Ibooks in the world. This despite the fact life? There are many examples in the that only a small number of people adopt history of our country where such changes Netaji National Reading Promotion writing as a profession in our country. were brought about by words. India will Recognition Award The strength of a country’s defence, nurture children from their early years 2 health, transport and communication, and the youth in a way that they will Azamgarh Book Fair which are seen as symbols of a country’s be able to become ambassadors of its 2 empowerment becomes meaningful, only literature of a twenty-first century India. if its people are able to express their For every Indian to be a “world citizen”, it In Conversation 3 achievements, aspirations and hopes in is imperative that the voice of the country their own words and languages. How does is heard in their own language on a global Lifetime Achievement Award the reading of a book or an event bring stage in a structured manner. to NBT 3 NBT Books on and by Women 4-5 Excerpts 6 Celebrate World Wildlife Day with NBT Books 7 PICK OF THE MONTH Pleasures of Reading Prem Pal Sharma Translation: Arpita Sen ISBN 978-237-81-9523-2; Rs 175 MARCH 2021 NBT NEWSLETTER 1 As you may be aware, on 31 January Prime Minister will create a large number society rooted in the objectives of the 2021 in his weekly broadcast programme of young writers, who will able to express National Education Policy-2020, will of “Mann Ki Baat”, the Prime Minister themselves in their own mother tongue promote Indian languages and their said, “In every corner of the land, and their writings will be promoted with literatures among the youth. -

Kimberley Marine Biota. Historical Data: Marine Plants
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 84 045–067 (2014) DOI: 10.18195/issn.0313-122x.84.2014.045-067 SUPPLEMENT Kimberley marine biota. Historical data: marine plants John M. Huisman1,2* and Alison Sampey3 1 Western Australian Herbarium, Science Division, Department of Parks and Wildlife, Locked Bag 104, Bentley DC, Western Australian 6983, Australia. 2 School of Veterinary and Life Sciences, Murdoch University, Murdoch, Western Australian 6150, Australia. 3 Department of Aquatic Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australian 6986, Australia. * Email: [email protected] ABSTRACT – Here, we document 308 species of marine flora from the Kimberley region of Western Australia based on collections held in the Western Australian Herbarium and on reports on marine biodiversity surveys to the region. Included are 12 species of seagrasses, 18 species of mangrove and 278 species of marine algae. Seagrasses and mangroves in the region have been comparatively well surveyed and their taxonomy is stable, so it is unlikely that further species will be recorded. However, the marine algae have been collected and documented only more recently and it is estimated that further surveys will increase the number of recorded species to over 400. The bulk of the marine flora comprised widespread Indo-West Pacific species, but there were also many endemic species with more endemics reported from the inshore areas than the offshore atolls. This number also will increase with the description of new species from the region. Collecting across the region has been highly variable due to the remote location, logistical difficulties and resource limitations. -

Rare Plants of Louisiana
Rare Plants of Louisiana Agalinis filicaulis - purple false-foxglove Figwort Family (Scrophulariaceae) Rarity Rank: S2/G3G4 Range: AL, FL, LA, MS Recognition: Photo by John Hays • Short annual, 10 to 50 cm tall, with stems finely wiry, spindly • Stems simple to few-branched • Leaves opposite, scale-like, about 1mm long, barely perceptible to the unaided eye • Flowers few in number, mostly born singly or in pairs from the highest node of a branchlet • Pedicels filiform, 5 to 10 mm long, subtending bracts minute • Calyx 2 mm long, lobes short-deltoid, with broad shallow sinuses between lobes • Corolla lavender-pink, without lines or spots within, 10 to 13 mm long, exterior glabrous • Capsule globe-like, nearly half exerted from calyx Flowering Time: September to November Light Requirement: Full sun to partial shade Wetland Indicator Status: FAC – similar likelihood of occurring in both wetlands and non-wetlands Habitat: Wet longleaf pine flatwoods savannahs and hillside seepage bogs. Threats: • Conversion of habitat to pine plantations (bedding, dense tree spacing, etc.) • Residential and commercial development • Fire exclusion, allowing invasion of habitat by woody species • Hydrologic alteration directly (e.g. ditching) and indirectly (fire suppression allowing higher tree density and more large-diameter trees) Beneficial Management Practices: • Thinning (during very dry periods), targeting off-site species such as loblolly and slash pines for removal • Prescribed burning, establishing a regime consisting of mostly growing season (May-June) burns Rare Plants of Louisiana LA River Basins: Pearl, Pontchartrain, Mermentau, Calcasieu, Sabine Side view of flower. Photo by John Hays References: Godfrey, R. K. and J. W. Wooten. -

Proceedings of a Workshop for Monitoring
Seagrass-Watch Proceedings of a Workshop for Monitoring Seagrass Habitats in the Kimberley Region, Western Australia Department of Environment & Conservation - West Kimberly Office Broome, Western Australia 23-24 August 2009 Len McKenzie & Rudi Yoshida First Published 2009 ©Seagrass-Watch HQ, 2009 Copyright protects this publication. Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Disclaimer Information contained in this publication is provided as general advice only. For application to specific circumstances, professional advice should be sought. Seagrass-Watch HQ has taken all reasonable steps to ensure the information contained in this publication is accurate at the time of the survey. Readers should ensure that they make appropriate enquires to determine whether new information is available on the particular subject matter. The correct citation of this document is McKenzie, LJ & Yoshida, R.L. (2009). Seagrass-Watch: Proceedings of a Workshop for Monitoring Seagrass Habitats in the Kimberley Region, Western Australia. Department of Environment & Conservation - West Kimberley Office, Broome, 23 - 24 August 2009. (Seagrass-Watch HQ, Cairns). 58pp. Produced by Seagrass-Watch HQ Front cover photos (left to right) Town Beach Broome, One Arm Creek and -

1 Phylogenetic Regionalization of Marine Plants Reveals Close Evolutionary Affinities Among Disjunct Temperate Assemblages Barna
Phylogenetic regionalization of marine plants reveals close evolutionary affinities among disjunct temperate assemblages Barnabas H. Darua,b,*, Ben G. Holtc, Jean-Philippe Lessardd,e, Kowiyou Yessoufouf and T. Jonathan Daviesg,h aDepartment of Organismic and Evolutionary Biology and Harvard University Herbaria, Harvard University, Cambridge, MA 02138, USA bDepartment of Plant Science, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa cDepartment of Life Sciences, Imperial College London, Silwood Park Campus, Ascot SL5 7PY, United Kingdom dQuebec Centre for Biodiversity Science, Department of Biology, McGill University, Montreal, QC H3A 0G4, Canada eDepartment of Biology, Concordia University, Montreal, QC, H4B 1R6, Canada; fDepartment of Environmental Sciences, University of South Africa, Florida campus, Florida 1710, South Africa gDepartment of Biology, McGill University, Montreal, QC H3A 0G4, Canada hAfrican Centre for DNA Barcoding, University of Johannesburg, PO Box 524, Auckland Park, Johannesburg 2006, South Africa *Corresponding author Email: [email protected] (B.H. Daru) Running head: Phylogenetic regionalization of seagrasses 1 Abstract While our knowledge of species distributions and diversity in the terrestrial biosphere has increased sharply over the last decades, we lack equivalent knowledge of the marine world. Here, we use the phylogenetic tree of seagrasses along with their global distributions and a metric of phylogenetic beta diversity to generate a phylogenetically-based delimitation of marine phytoregions (phyloregions). We then evaluate their evolutionary affinities and explore environmental correlates of phylogenetic turnover between them. We identified 11 phyloregions based on the clustering of phylogenetic beta diversity values. Most phyloregions can be classified as either temperate or tropical, and even geographically disjunct temperate regions can harbor closely related species assemblages. -

Library Catalogue
Id Access No Title Author Category Publisher Year 1 9277 Jawaharlal Nehru. An autobiography J. Nehru Autobiography, Nehru Indraprastha Press 1988 historical, Indian history, reference, Indian 2 587 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence historical, Indian history, reference, Indian 3 605 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence 4 3633 Jawaharlal Nehru. Rebel and Stateman B. R. Nanda Biography, Nehru, Historical Oxford University Press 1995 5 4420 Jawaharlal Nehru. A Communicator and Democratic Leader A. K. Damodaran Biography, Nehru, Historical Radiant Publlishers 1997 Indira Gandhi, 6 711 The Spirit of India. Vol 2 Biography, Nehru, Historical, Gandhi Asia Publishing House 1975 Abhinandan Granth Ministry of Information and 8 454 Builders of Modern India. Gopal Krishna Gokhale T.R. Deogirikar Biography 1964 Broadcasting Ministry of Information and 9 455 Builders of Modern India. Rajendra Prasad Kali Kinkar Data Biography, Prasad 1970 Broadcasting Ministry of Information and 10 456 Builders of Modern India. P.S.Sivaswami Aiyer K. Chandrasekharan Biography, Sivaswami, Aiyer 1969 Broadcasting Ministry of Information and 11 950 Speeches of Presidente V.V. Giri. Vol 2 V.V. Giri poitical, Biography, V.V. Giri, speeches 1977 Broadcasting Ministry of Information and 12 951 Speeches of President Rajendra Prasad Vol. 1 Rajendra Prasad Political, Biography, Rajendra Prasad 1973 Broadcasting Eminent Parliamentarians Monograph Series. 01 - Dr. Ram Manohar 13 2671 Biography, Manohar Lohia Lok Sabha 1990 Lohia Eminent Parliamentarians Monograph Series. 02 - Dr. Lanka 14 2672 Biography, Lanka Sunbdaram Lok Sabha 1990 Sunbdaram Eminent Parliamentarians Monograph Series. 04 - Pandit Nilakantha 15 2674 Biography, Nilakantha Lok Sabha 1990 Das Eminent Parliamentarians Monograph Series. -

Seagrasses from the Philippines
SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES •NUMBER 21 Seagrasses from the Philippines Ernani G. Mefiez, Ronald C. Phillips, and Hilconida P. Calumpong ISSUED DEC 11983 SMITHSONIAN PUBLICATIONS SMITHSONIAN INSTITUTION PRESS City of Washington 1983 ABSTRACT Menez, Ernani G., Ronald C. Phillips, and Hilconida P. Calumpong. Sea grasses from the Philippines. Smithsonian Contributions to the Marine Sciences, number 21, 40 pages, 26 figures, 1983.—Seagrasses were collected from various islands in the Philippines during 1978-1982. A total of 12 species in seven genera are recorded. Generic and specific keys, based on vegetative characters, are provided for easier differentiation of the seagrasses. General discussions of seagrass biology, ecology, collection and preservation are presented. Local and world distribution of Philippine seagrasses are also included. OFFICIAL PUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution's annual report, Smithsonian Year. SERIES COVER DESIGN: Seascape along the Atlantic coast of eastern North America. Library of Congress Cataloging in Publication Data Menez, Ernani G. Seagrasses from the Philippines. (Smithsonian contributions to the marine sciences ; no. 21) Bibliography: p. Supt. of Docs, no.: SI 1.41:21 1. Seagrasses—Philippines. I. Phillipps, Ronald C. II. Calumpong, Hilconida P. III. Ti tle. IV. Series. QK495.A14M46 1983 584.73 83-600168 Contents Page Introduction 1 Acknowledgments 3 Materials and Methods 3 Collecting and Preserving Seagrasses 4 General Features of Seagrass Biology and Ecology 6 Key to the Philippine Seagrasses 7 Division ANTHOPHYTA 8 Class MONOCOTYLEDONEAE 8 Order HELOBIAE 8 Family POTAMOGETONACEAE 8 Cymodocea rotundata Ehrenberg and Hemprich, ex Ascherson 8 Cymodocea serrulata (R.