Template B V3.0 (Beta): Created by J. Nail 06/2015 Evaluation of Kudzu Bug As a Pest in Mississippi Soybean Production Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Behind the Scenes, I Have Been Watching the Bowerbird Record
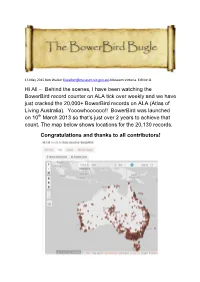
15 May 2015 Ken Walker ([email protected]) Museum Victoria. Edition 8. Hi All – Behind the scenes, I have been watching the BowerBird record counter on ALA tick over weekly and we have just cracked the 20,000+ BowerBird records on ALA (Atlas of Living Australia). Yooowhoooooo!! BowerBird was launched on 10th March 2013 so that’s just over 2 years to achieve that count. The map below shows locations for the 20,130 records. Congratulations and thanks to all contributors! From a purely funding analysis point of view, the initial ALA grant to develop BowerBird was $350,000 which at 20,130 records is currently returning per record at a cost of $17.38 and that per record cost will only get lower as more BowerBird records are added and uploaded. For some species on ALA, BowerBird provides the only distributional species data points: For other species on ALA, BowerBird provides the only species image for species on ALA: From a Biosecurity point of view of tracking exotic species, BowerBird has supplied all records post 2007 for the exotic South African Carder bee. All of the blue dots represent BowerBird records. Other than the great biodiversity results that BowerBird delivers through ALA, there are a myriad of other intangible benefits that come from the BowerBird website. Intangibles such as the member’s conversations, identification discussions, the friendships, the biological statements and the generated innate knowledge. One particular “intangible”, I would like to tell you about here. The PBCRC (Plant Biosecurity, Cooperative Research Centre) has a theme called “Building Resilience through Remote Indigenous Engagement”. -

Kudzu Vine Distribution of Kudzu
Kudzu Bug Megacopta cribraria Photo: ©2007 Charles Lam, Wikimedia commons Kudzu Bug • First species of Plataspidae family in Western Hemisphere! • 2009: First detection in U.S. (Georgia) • Agricultural pest to legumes • Nuisance pest to homeowners Photos: (Top) - Michasia Harris, University of Georgia, Bugwood.org #5474627; (Bottom) - Daniel R. Suiter, University of Georgia, Bugwood.org # 5407722 Kudzu Bug Distribution No sampling Sampled but not found Intercepted or detected, but not established Established by survey or consensus Map courtesy of Pest Tracker, National Agricultural Pest Information System (NAPIS), and The University of Georgia - Center for Invasive Species and Ecosystem Health, eddmaps.org Photo: Russ Ottens, University of Georgia, Bugwood.org #5475145 Susceptible Plants In the United States: • Kudzu • Soybean and other legumes • Corn • Sweet potato • Wisteria • Wheat In native regions: • NOT an agricultural pest Kudzu bug on soybean Photo: Michasia Harris, University of Georgia, Bugwood.org #5473918 Kudzu “The vine that ate the South” Kudzu vine Distribution of Kudzu Photo: Leslie J. Mehrhoff, University of Connecticut, Bugwood.org #5483399; Map courtesy of USDA database, National Agricultural Pest Information System (NAPIS) Identification Eggs Nymphs Symbiont capsules in egg mass Hairy appearance of nymphs Photos: (Top) - Joe Eger, Dow AgroSciences, Bugwood.org #5471831; Yasmin Cardoza, North Carolina State University, Bugwood.org #5472919 (Bottom) - Yasmin Cardoza, North Carolina State University, Bugwood.org #5472911; -

Your HATCH Project Sample Title Here
HATCH PROJECT Alabama Agricultural Experiment Station Auburn University TITLE OF PROPOSED PROJECT: Development and Implementation of Alternative Pest Management Strategies for Emerging Crop Pests in Alabama PRINCIPAL INVESTIGATOR: Department: 1 SUMMARY OF CRIS DATABASE SEARCH A search of the Current Research Information System (CRIS) revealed a total of eight projects relating to arthropod pest management. Entering “insect pest management” returned 6 matches, while entering “insect pest management in fruit/vegetable/specialty crops” returned 2 matches. Four of these are Hatch projects and only two are vaguely related to the focus of this Hatch project: 1) Development and Implementation of New Reduced-Risk Insect Management Strategies for Blueberries and Cranberries - by ??, ?? University, 2) Insect Pest Management of Sweet Potato and Vegetable Crops – by :??, ?? University, and 3) High Value Specialty Crop Pest Management – by ??, ?? University. None of the projects directly addresses the specific objectives of or overlaps with this proposed Hatch project. NON TECHNICAL SUMMARY This project focuses on the management of key emerging pests of crops in Alabama, specifically pests of fruit and vegetable crops and soybean. Fruit and vegetable crops constitute an important group of horticultural crops in the U.S. with an annual market value of approximately $23 billion. Soybean is also an important crop in the U.S with an annual market value of about $42 billion. Several arthropod pests attack these crops in Alabama with the potential to cause significant economic losses to producers. The goal of this project is to develop and implement ecologically based and cost-effective integrated pest management (IPM) practices for major and emerging pests of peaches, cucurbits, crucifers and soybean. -

EPPO Reporting Service
ORGANISATION EUROPEENNE EUROPEAN AND MEDITERRANEAN ET MEDITERRANEENNE PLANT PROTECTION POUR LA PROTECTION DES PLANTES ORGANIZATION EPPO Reporting Service NO. 09 PARIS, 2014-09-01 CONTENTS _______________________________________________________________________ Pests & Diseases 2014/158 - New additions to the EPPO A1 and A2 Lists 2014/159 - PQR - the EPPO database on quarantine pests: new update 2014/160 - EPPO Global Database has just been launched (https://gd.eppo.int/) 2014/161 - Megacopta cribraria is an emerging pest of soybean in the USA: addition to the EPPO Alert List 2014/162 - Interception of Callidiellum villosulum in Malta 2014/163 - A new disease of coast live oaks (Quercus agrifolia) in California (US) is associated with Geosmithia pallida and Pseudopityophthorus pubipennis 2014/164 - First report of Globodera pallida in Bosnia and Herzegovina 2014/165 - First report of Xanthomonas fragariae in Mexico 2014/166 - Studies on olive (Olea europaea) as a host of Xylella fastidiosa in California (US) 2014/167 - First reports of ‘Candidatus Liberibacter asiaticus’ in Guadeloupe and Martinique 2014/168 - Tomato apical stunt viroid detected on tomatoes in Italy 2014/169 - Survey on Potato spindle tuber viroid and Tomato apical stunt viroid in ornamental plants in Croatia. 2014/170 - First report of Fusarium oxysporum f. sp. cubense tropical race 4 in Jordan 2014/171 - New data on quarantine pests and pests of the EPPO Alert List CONTENTS ___________________________________________________________________________ Invasive Plants 2014/172 -

Current Status of the Kudzu Bug, Megacopta Cribraria, in North America
Presentation to: Annual Meeting of the National Plant Board Current Status of the Kudzu Bug, Megacopta Mystic, Connecticut Acknowledgments 24 July 2012 cribraria, in North America Wayne A. Gardner, Professor Department of Entomology John All University of Georgia University of Georgia Lisa Ames Georgia Dept of Agriculture Griffin Campus Chuck Bargeron Emory University Griffin, Georgia 30223 USA David Buntin USDA Forest Service 770‐228‐7341 Keith Douce USDA‐ARS [email protected] Wayne Gardner USDA‐APHIS‐PPQ Jim Hanula Clemson UiUnivers ity Scott Horn NC State University Tracie Jenkins NC Dept of Agriculture Robert Kemerait Wingate University Joseph LaForest Virginia Tech Hal Peeler University of Georgia Auburn University Phillip Roberts College of Agricultural and Environmental Sciences Megacopta Working Group Dow AgroScience John Ruberson Florida Dept of Agriculture Paul Smith Mississippi State University Alton (Stormy) Sparks, Jr. Dan Suiter Clay Talton Michael Toews Yanzhou Zhang The Insect Megacopta cribraria Initial Discovery Hemiptera: Plataspidae October 2009 A Development time from egg to Samples submitted to the UGA adult = 24 to 56 days. Homeowner Insect & Weed Diagnostics Laboratory. Numbers of eggs produced per female = 26 to 274 with 15 eggs per egg mass. October 28, 2009: Site visit to Jackson Co., GA, thousands of Eggs usually deposited in 2‐3 adult kudzu bugs on homes. parallel rows stuck black Upper images provided by Jeremy Greene, Clemson University substance deposited by female. Kudzu growing 30 m from homes harbored large numbers 5 nymphal instars. of adults and some late‐instar nymphs. Adult longevity = 23 to 77 days. Overwinter as adults in groups Adults seeking overwintering usually under debris or under sites at the homes. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

The Maryland Entomologist
THE MARYLAND ENTOMOLOGIST Insect and related-arthropod studies in the Mid-Atlantic region Volume 6, Number 2 September 2014 September 2014 The Maryland Entomologist Volume 6, Number 2 MARYLAND ENTOMOLOGICAL SOCIETY www.mdentsoc.org Executive Committee: Co-Presidents Timothy Foard and Frederick Paras Vice President Philip J. Kean Secretary Richard H. Smith, Jr. Treasurer Edgar A. Cohen, Jr. Historian (vacant) Publications Editor Eugene J. Scarpulla The Maryland Entomological Society (MES) was founded in November 1971, to promote the science of entomology in all its sub-disciplines; to provide a common meeting venue for professional and amateur entomologists residing in Maryland, the District of Columbia, and nearby areas; to issue a periodical and other publications dealing with entomology; and to facilitate the exchange of ideas and information through its meetings and publications. The MES was incorporated in April 1982 and is a 501(c)(3) non-profit, scientific organization. The MES logo features an illustration of Euphydryas phaëton (Drury) (Lepidoptera: Nymphalidae), the Baltimore Checkerspot, with its generic name above and its specific epithet below (both in capital letters), all on a pale green field; all these are within a yellow ring double-bordered by red, bearing the message “● Maryland Entomological Society ● 1971 ●”. All of this is positioned above the Shield of the State of Maryland. In 1973, the Baltimore Checkerspot was named the official insect of the State of Maryland through the efforts of many MES members. Membership in the MES is open to all persons interested in the study of entomology. All members receive the annual journal, The Maryland Entomologist, and the monthly e-newsletter, Phaëton. -

Great Lakes Entomologist the Grea T Lakes E N Omo L O G Is T Published by the Michigan Entomological Society Vol
The Great Lakes Entomologist THE GREA Published by the Michigan Entomological Society Vol. 45, Nos. 3 & 4 Fall/Winter 2012 Volume 45 Nos. 3 & 4 ISSN 0090-0222 T LAKES Table of Contents THE Scholar, Teacher, and Mentor: A Tribute to Dr. J. E. McPherson ..............................................i E N GREAT LAKES Dr. J. E. McPherson, Educator and Researcher Extraordinaire: Biographical Sketch and T List of Publications OMO Thomas J. Henry ..................................................................................................111 J.E. McPherson – A Career of Exemplary Service and Contributions to the Entomological ENTOMOLOGIST Society of America L O George G. Kennedy .............................................................................................124 G Mcphersonarcys, a New Genus for Pentatoma aequalis Say (Heteroptera: Pentatomidae) IS Donald B. Thomas ................................................................................................127 T The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Missouri Robert W. Sites, Kristin B. Simpson, and Diane L. Wood ............................................134 Tymbal Morphology and Co-occurrence of Spartina Sap-feeding Insects (Hemiptera: Auchenorrhyncha) Stephen W. Wilson ...............................................................................................164 Pentatomoidea (Hemiptera: Pentatomidae, Scutelleridae) Associated with the Dioecious Shrub Florida Rosemary, Ceratiola ericoides (Ericaceae) A. G. Wheeler, Jr. .................................................................................................183 -

Invasive Stink Bugs and Related Species (Pentatomoidea) Biology, Higher Systematics, Semiochemistry, and Management
Invasive Stink Bugs and Related Species (Pentatomoidea) Biology, Higher Systematics, Semiochemistry, and Management Edited by J. E. McPherson Front Cover photographs, clockwise from the top left: Adult of Piezodorus guildinii (Westwood), Photograph by Ted C. MacRae; Adult of Murgantia histrionica (Hahn), Photograph by C. Scott Bundy; Adult of Halyomorpha halys (Stål), Photograph by George C. Hamilton; Adult of Bagrada hilaris (Burmeister), Photograph by C. Scott Bundy; Adult of Megacopta cribraria (F.), Photograph by J. E. Eger; Mating pair of Nezara viridula (L.), Photograph by Jesus F. Esquivel. Used with permission. All rights reserved. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2018 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed on acid-free paper International Standard Book Number-13: 978-1-4987-1508-9 (Hardback) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materi- als or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, micro- filming, and recording, or in any information storage or retrieval system, without written permission from the publishers. -

Centomologica: -'F
:1 |II || ISSN 0001-561X AdTA| CENTOMOLOGICA: -'F. NNICA I A:_:1 $-** ; R. E. Linn vuori | Heteortera of Yemen and Siouth Yemenll 0 ,~~~~~~~~~~~~~~~~~~~I Vo41.4 1989 : ANNALES ENTOMOLOGICI FEMNNICI ACTA ENTOMOLOGICA FENNICA Published since 1935, four numbers a year. Published since 1947, monographs Annual subscription FIM 150, in Finland at irregular intervals. FIM 120. Price variable. Address: Zoological Museum, P. Rautatiek. 13, SF-00100 Helsinki, Finland. Publishers Suomen Hy6nteistieteellinen Seura Entomological Society of Finland - Societas Entomologica Fennica Entomologiska Foreningen i Helsingfors - Helsingin Hyonteistieteellinen Yhdistys Societas Entomologica Helsingforsiensis Editorial Board Chairman: A. Jansson (chief editor) Other members: K. Heliovaara (assistant editor of Acta), L. Hulddn (secretary, assistant editor), R. livarinen (treasurer), H. Krogerus, i. Mannerkoski, H. Silfverberg (editor of Acta) Board of Trustees President: E. Kangas Other members: 0. Bistrom, 1. Terds, A. Pekkarinen, R. Rosengren (vice president) Annales Entomologici Fennici publishes scientific papers, notes and reviews based principally on Finnish entomological investigations. Monographs and other longer articles are directed to Acta Entomologica Fennica, articles of mainly Nordic interest to Notulae Entomologicae. Contributors are requested to take into consideration the style and format of articles in recently published volumes. Two copies of each manuscript must be submitted with the original. As modern techniques often allow printing directly from computer diskettes, the editor should be informed if the manuscript is written on a word processor. The journals are cited selectively by Bibliographie der Pflanzenschutz-Literatur of Biologische Bundesanstaft for Land- und Forstwirtschaft, Biological Abstracts of the Biosciences Information Service, Current Contents (Series Agriculture, Biology & Environmental Sciences) of Institute for Scientific Information, Entomology Abstracts of Information Retrieval Limited, and Review of Applied Entomology (Series A. -
ESA 2 0 14 9-12 March 2014 Des Moines, Iowa 2014 NCB-ESA Corporate Sponsors CONTENTS
NCB ESA 2 0 14 9-12 March 2014 Des Moines, Iowa 2014 NCB-ESA Corporate Sponsors CONTENTS Meeting Logistics ....................................................1 2014 NCB-ESA Officers and Committees .................5 2014 Award Recipients ...........................................7 Sunday, 9 March 2014 At-a-Glance ..................................................18 Afternoon .....................................................19 Monday, 10 March 2014 At-a-Glance ..................................................23 Posters .........................................................25 Morning .......................................................30 Afternoon .....................................................35 Tuesday, 11 March 2014 At-a-Glance ..................................................45 Posters .........................................................47 Morning .......................................................51 Afternoon .....................................................55 Wednesday, 12 March 2014 At-a-Glance ..................................................60 Morning .......................................................61 Author Index ........................................................67 Scientific Name Index ...........................................77 Keyword Index ......................................................82 Common Name Index ...........................................83 Map of Meeting Facilities ..............inside back cover i MEETING LOGISTICS Registration All participants must register -

Molecular Phylogeny of Trissolcus Wasps, Natural Enemies of Stink Bugs 201 Doi: 10.3897/Jhr.73.39563 RESEARCH ARTICLE
JHR 73: 201–217 (2019)Molecular phylogeny of Trissolcus wasps, natural enemies of stink bugs 201 doi: 10.3897/jhr.73.39563 RESEARCH ARTICLE http://jhr.pensoft.net Molecular phylogeny of Trissolcus wasps (Hymenoptera, Scelionidae) associated with Halyomorpha halys (Hemiptera, Pentatomidae) Elijah J. Talamas1,4, Marie-Claude Bon2, Kim A. Hoelmer3, Matthew L. Buffington4 1 Florida State Collection of Arthropods, Division of Plant Industry, Florida Department of Agriculture and Consumer Services, Gainesville, FL, USA 2 European Biological Control Laboratory, USDA/ARS, Montpellier, France 3 Beneficial Insects Introduction Research Unit, USDA/ARS, Newark, DE, USA 4 Systematic Entomo- logy Laboratory, USDA/ARS c/o NMNH, Smithsonian Institution, Washington DC, USA Corresponding author: Elijah J. Talamas ([email protected]) Academic editor: G. Broad | Received 29 August 2019 | Accepted 1 November 2019 | Published 18 November 2019 http://zoobank.org/4370473A-EA58-42C9-B1AF-3CBFDDD3C65F Citation: Talamas EJ, Bon M-C, Hoelmer KA, Buffington ML (2019) Molecular phylogeny ofTrissolcus wasps (Hymenoptera, Scelionidae) associated with Halyomorpha halys (Hemiptera, Pentatomidae). In: Talamas E (Eds) Advances in the Systematics of Platygastroidea II. Journal of Hymenoptera Research 73: 201–217. https://doi. org/10.3897/jhr.73.39563 Abstract As the brown marmorated stink bug (Halyomorpha halys) has spread across the Northern Hemisphere, re- search on its egg parasitoids has increased accordingly. These studies have included species-level taxonomy, experimental assessments of host ranges in quarantine, and surveys to assess parasitism in the field. We here present a molecular phylogeny of Trissolcus that includes all species that have been reared from live H. halys eggs. Species-group concepts are discussed and revised in the light of the phylogenetic analyses.